Abstract
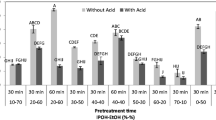
Hydrogen, volatile fatty acids (VFAs), and methane coproduction from sweet sorghum stems using bacterial consortium was investigated as an efficient and sustainable pre-treatment strategy to improve energy recovery. Integrated two-stage dark fermentation and methanization approach aimed to reduce fractionation, juice extraction, and pre-treatment steps to improve the efficiency and sustainability of stalks energy bioconversion. Stems biomass loading did not significantly influence hydrogen and VFAs productivities. Energy recovery yields were (7.07) and (10.01) MJ/kg dry matter (DM), respectively, for raw stem single dark fermentation (DF) and methanization processes, corresponding to 41.22% and 58.37% of raw stalk energy potential. Methanogenic potential increase of 31.9% and energy bioconversion yield of 13.21 MJ/kg DM were reached for solid residues from DF (80.75% of their energy content), suggesting that bacterial consortium efficiently pre-treated sorghum stalk fibers. Coupling process led to 88.74% net biomass energy recovery yield, corresponding respectively to 57.38% and 40.23% more than single DF and methanization. Fiber degradation ability of DF bacterial consortium significantly contributed to improve sorghum stalk energy recovery efficiency and cost-competitiveness.
Graphical Abstract







Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Islam MS, Zhang Z, Qu S-B et al (2021) Coproduction of hydrogen and volatile fatty acids via integrated two-step fermentation of sweet sorghum stalks by alkaline and enzymatic treatment. Biomass Bioenergy 145:105923. https://doi.org/10.1016/j.biombioe.2020.105923
Chen J, Huang S, Kamran HW (2023) Empowering sustainability practices through energy transition for sustainable development goal 7: the role of energy patents and natural resources among European Union economies through advanced panel. Energy Policy 176:113499. https://doi.org/10.1016/j.enpol.2023.113499
Agrawal R, Verma A, Singhania RR et al (2021) Current understanding of the inhibition factors and their mechanism of action for the lignocellulosic biomass hydrolysis. Bioresour Technol 332:125042. https://doi.org/10.1016/j.biortech.2021.125042
Liu Z, Li H, Gao X et al (2022) Rational highly dispersed ruthenium for reductive catalytic fractionation of lignocellulose. Nat Commun 13:4716. https://doi.org/10.1038/s41467-022-32451-5
Basak B, Kumar R, Bharadwaj AVSLS et al (2023) Advances in physicochemical pretreatment strategies for lignocellulose biomass and their effectiveness in bioconversion for biofuel production. Bioresour Technol 369:128413. https://doi.org/10.1016/j.biortech.2022.128413
Sampath P, Brijesh RKR et al (2020) Biohydrogen production from organic waste – a review. Chem Eng Technol 43:1240–1248. https://doi.org/10.1002/ceat.201900400
Sillero L, Sganzerla WG, Forster-Carneiro T et al (2022) A bibliometric analysis of the hydrogen production from dark fermentation. Int J Hydrog Energy 47:27397–27420. https://doi.org/10.1016/j.ijhydene.2022.06.083
Fuess LT, Dos Santos GM, Delforno TP et al (2020) Biochemical butyrate production via dark fermentation as an energetically efficient alternative management approach for vinasse in sugarcane biorefineries. Renew Energy 158:3–12. https://doi.org/10.1016/j.renene.2020.05.063
Parvathy Eswari A, Ravi YK, Kavitha S, Rajesh Banu J (2023) Recent insight into anaerobic digestion of lignocellulosic biomass for cost effective bioenergy generation. E-Prime - Adv Electr Eng Electron Energy 3:100119. https://doi.org/10.1016/j.prime.2023.100119
Awogbemi O, Kallon DVV (2022) Pretreatment techniques for agricultural waste. Case Stud Chem Environ Eng 6:100229. https://doi.org/10.1016/j.cscee.2022.100229
Timofeeva SS, Karaeva JV, Kovalev AA et al (2023) Steam gasification of digestate after anaerobic digestion and dark fermentation of lignocellulosic biomass to produce syngas with high hydrogen content. Int J Hydrog Energy 48:7559–7568. https://doi.org/10.1016/j.ijhydene.2022.11.260
Hamadou B, Djomdi D, Zieba Falama R et al (2023) Optimization of energy recovery efficiency from sweet sorghum stems by ethanol and methane fermentation processes coupling. Bioengineered 14:228–244. https://doi.org/10.1080/21655979.2023.2234135
Tang W, Wu X, Huang C et al (2021) Natural surfactant-aided dilute sulfuric acid pretreatment of waste wheat straw to enhance enzymatic hydrolysis efficiency. Bioresour Technol 324:124651. https://doi.org/10.1016/j.biortech.2020.124651
Haldar D, Purkait MK (2021) A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: mechanistic insight and advancements. Chemosphere 264:128523. https://doi.org/10.1016/j.chemosphere.2020.128523
Wong JL, Khadaroo SNBA, Cheng JLY et al (2023) Green solvent for lignocellulosic biomass pretreatment: an overview of the performance of low transition temperature mixtures for enhanced bio-conversion. Mater 1:100012. https://doi.org/10.1016/j.nxmate.2023.100012
Bakari H, Djomdi, Falama Ruben Z, et al (2023) Optimization of bioethanol production after enzymatic treatment of sweet sorghum stalks. Waste Biomass Valorizationhttps://doi.org/10.1007/s12649-022-02026-y
Paramasivan S, Sankar S, Senthil Velavan R et al (2021) Assessing the potential of lignocellulosic energy crops as an alternative resource for bioethanol production using ultrasound assisted dilute acid pretreatment. Mater Today Proc 45:3279–3285. https://doi.org/10.1016/j.matpr.2020.12.470
Wang R, Wang K, Zhou M et al (2021) Efficient fractionation of moso bamboo by synergistic hydrothermal-deep eutectic solvents pretreatment. Bioresour Technol 328:124873. https://doi.org/10.1016/j.biortech.2021.124873
Ling R, Wu W, Yuan Y et al (2021) Investigation of choline chloride-formic acid pretreatment and Tween 80 to enhance sugarcane bagasse enzymatic hydrolysis. Bioresour Technol 326:124748. https://doi.org/10.1016/j.biortech.2021.124748
Li W, Shen Y, Liu H et al (2023) Bioconversion of lignocellulosic biomass into bacterial nanocellulose: challenges and perspectives. Green Chem Eng 4:160–172. https://doi.org/10.1016/j.gce.2022.04.007
Noblecourt A, Christophe G, Larroche C et al (2017) High hydrogen production rate in a submerged membrane anaerobic bioreactor. Int J Hydrog Energy 42:24656–24666. https://doi.org/10.1016/j.ijhydene.2017.08.037
Ribeiro T, Cresson R, Pommier S et al (2020) Measurement of biochemical methane potential of heterogeneous solid substrates: results of a two-phase French inter-laboratory study. Water 12:2814. https://doi.org/10.3390/w12102814
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Latimer GW (2023) Official methods of analysis of AOAC International, 22nd ed. Oxford University PressNew York. https://doi.org/10.1093/9780197610145.001.0001
Soest PJV (1963) Use of detergents in the analysis of fibrous feeds. II. A Rapid Method for the Determination of Fiber and Lignin. J AOAC Int 46:829–835. https://doi.org/10.1093/jaoac/46.5.829
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text Res J 29:786–794. https://doi.org/10.1177/004051755902901003
Feng Y (2022) Effects of different concentrations of sorghum straw on hydrogen production by oscillating photosynthetic organisms. In AIP Conference Proceedings. AIP Publishing LLC. Xi’an, China, 2474:020020. https://doi.org/10.1063/5.0079072
Ntaikou I, Gavala HN, Kornaros M, Lyberatos G (2008) Hydrogen production from sugars and sweet sorghum biomass using Ruminococcus albus. Int J Hydrog Energy 33:1153–1163. https://doi.org/10.1016/j.ijhydene.2007.10.053
Noblecourt A, Christophe G, Larroche C, Fontanille P (2018) Hydrogen production by dark fermentation from pre-fermented depackaging food wastes. Bioresour Technol 247:864–870. https://doi.org/10.1016/j.biortech.2017.09.199
Antonopoulou G, Gavala HN, Skiadas IV, Lyberatos G (2010) Influence of pH on fermentative hydrogen production from sweet sorghum extract. Int J Hydrog Energy 35:1921–1928. https://doi.org/10.1016/j.ijhydene.2009.12.175
Panagiotopoulos IA, Bakker RR, De Vrije T et al (2010) Pretreatment of sweet sorghum bagasse for hydrogen production by Caldicellulosiruptor saccharolyticus. Int J Hydrog Energy 35:7738–7747. https://doi.org/10.1016/j.ijhydene.2010.05.075
Wu Q, Bao X, Guo W et al (2019) Medium chain carboxylic acids production from waste biomass: current advances and perspectives. Biotechnol Adv 37:599–615. https://doi.org/10.1016/j.biotechadv.2019.03.003
Fontanille P, Kumar V, Christophe G et al (2012) Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Bioresour Technol 114:443–449. https://doi.org/10.1016/j.biortech.2012.02.091
Antonopoulou G, Gavala HN, Skiadas IV et al (2008) Biofuels generation from sweet sorghum: fermentative hydrogen production and anaerobic digestion of the remaining biomass. Bioresour Technol 99:110–119. https://doi.org/10.1016/j.biortech.2006.11.048
Nurika I, Shabrina EN, Azizah N et al (2022) Application of ligninolytic bacteria to the enhancement of lignocellulose breakdown and methane production from oil palm empty fruit bunches (OPEFB). Bioresour Technol Rep 17:100951. https://doi.org/10.1016/j.biteb.2022.100951
Tsegaye B, Balomajumder C, Roy P (2018) Biodegradation of wheat straw by Ochrobactrum oryzae BMP03 and Bacillus sp. BMP01 bacteria to enhance biofuel production by increasing total reducing sugars yield. Environ Sci Pollut Res 25:30585–30596. https://doi.org/10.1007/s11356-018-3056-1
Singh JK, Chaurasia B, Dubey A et al (2020) Biological characterization and instrumental analytical comparison of two biorefining pretreatments for water hyacinth (Eichhornia crassipes) biomass hydrolysis. Sustainability 13:245. https://doi.org/10.3390/su13010245
Tang Z, Wu C, Tang W et al (2023) Enhancing enzymatic saccharification of sunflower straw through optimal tartaric acid hydrothermal pretreatment. Bioresour Technol 385:129279. https://doi.org/10.1016/j.biortech.2023.129279
Jamaldheen SB, Sharma K, Rani A et al (2018) Comparative analysis of pretreatment methods on sorghum (Sorghum durra) stalk agrowaste for holocellulose content. Prep Biochem Biotechnol 48:457–464. https://doi.org/10.1080/10826068.2018.1466148
Nlandu H, Belkacemi K, Chorfa N et al (2020) Flax nanofibrils production via supercritical carbon dioxide pre-treatment and enzymatic hydrolysis. Can J Chem Eng 98:84–95. https://doi.org/10.1002/cjce.23596
Narayanaswamy N, Faik A, Goetz DJ, Gu T (2011) Supercritical carbon dioxide pretreatment of corn stover and switchgrass for lignocellulosic ethanol production. Bioresour Technol 102:6995–7000. https://doi.org/10.1016/j.biortech.2011.04.052
Zheng Y, Lin H-M, Tsao GT (1998) Pretreatment for cellulose hydrolysis by carbon dioxide explosion. Biotechnol Prog 14:890–896. https://doi.org/10.1021/bp980087g
Głąb L, Sowiński J, Chmielewska J et al (2019) Comparison of the energy efficiency of methane and ethanol production from sweet sorghum (Sorghum bicolor (L.) Moench) with a variety of feedstock management technologies. Biomass Bioenergy 129:105332. https://doi.org/10.1016/j.biombioe.2019.105332
Herrmann C, Heiermann M, Idler C (2011) Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioresour Technol 102:5153–5161. https://doi.org/10.1016/j.biortech.2011.01.012
Ostovareh S, Karimi K, Zamani A (2015) Efficient conversion of sweet sorghum stalks to biogas and ethanol using organosolv pretreatment. Ind Crops Prod 66:170–177. https://doi.org/10.1016/j.indcrop.2014.12.023
Brown RC, Brown TR (2014) Biorenewable resources: engineering new products from agriculture. John Wiley & Sons Inc, Hoboken, NJ, USA. https://doi.org/10.1002/9781118524985
Ruggeri B, Tommasi T, Sassi G (2010) Energy balance of dark anaerobic fermentation as a tool for sustainability analysis. Int J Hydrog Energy 35:10202–10211. https://doi.org/10.1016/j.ijhydene.2010.08.014
Perera KRJ, Ketheesan B, Gadhamshetty V, Nirmalakhandan N (2010) Fermentative biohydrogen production: Evaluation of net energy gain. Int J Hydrog Energy 35:12224–12233. https://doi.org/10.1016/j.ijhydene.2010.08.037
Ghimire A, Frunzo L, Pirozzi F et al (2015) A review on dark fermentative biohydrogen production from organic biomass: process parameters and use of by-products. Appl Energy 144:73–95. https://doi.org/10.1016/j.apenergy.2015.01.045
Singh N, Singhania RR, Nigam PS et al (2022) Global status of lignocellulosic biorefinery: challenges and perspectives. Bioresour Technol 344:126415. https://doi.org/10.1016/j.biortech.2021.126415
Yang E, Chon K, Kim K-Y et al (2023) Pretreatments of lignocellulosic and algal biomasses for sustainable biohydrogen production: recent progress, carbon neutrality, and circular economy. Bioresour Technol 369:128380. https://doi.org/10.1016/j.biortech.2022.128380
Soares JF, Confortin TC, Todero I et al (2020) Dark fermentative biohydrogen production from lignocellulosic biomass: technological challenges and future prospects. Renew Sustain Energy Rev 117:109484. https://doi.org/10.1016/j.rser.2019.109484
Rorke D, Gueguim Kana EB (2016) Biohydrogen process development on waste sorghum (Sorghum bicolor) leaves: optimization of saccharification, hydrogen production and preliminary scale up. Int J Hydrog Energy 41:12941–12952. https://doi.org/10.1016/j.ijhydene.2016.06.112
Acknowledgements
Authors would like to thank the “Centre Imagerie Cellulaire Santé (CICS), UCA PARTNER, 63000 Clermont-Ferrand” and “Institut de Chimie de Clermont-Ferrand (ICCF)—UMR 6296” for their collaboration on biomass FESEM scanning and X-ray diffraction analyses.
Funding
Hamadou Bakari’s research mobility was financially supported by the French government through its Eiffel excellence scholarship program (Campus France (D21–0000000051)).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Bakari Hamadou, Christine Gardarin, Christelle Blavignac, Djomdi Djomdi, and Ruben Zieba Falama. The first draft of the manuscript was written by Bakari Hamadou, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
Biomass loading did not significantly influence H2 and VFAs productivities.
DF bacterial consortium effectively pre-treated sorghum stem fibrous fractions.
DF solid residues had 31.9% higher methanogenic potential than raw stems.
DF and AD coupling process led to 88.74% of net energy production yield.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hamadou, B., Djomdi, D., Falama, R.Z. et al. Hydrogen and Fatty Acid Production by Dark Fermentation of Sweet Sorghum Stalks as an Efficient Pre-treatment for Energy Recovery Before Their Bioconversion into Methane. Bioenerg. Res. (2024). https://doi.org/10.1007/s12155-024-10724-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12155-024-10724-9