Abstract
The influence of ultrasonic pretreatment with specific energy input ranging from 25 to 550 kJ/kg volatile solids on the mixture of Sida hermaphrodita (L.) Rusby mixed with cattle manure disintegration and subsequent anaerobic digestion was assessed. The pretreatment process led to significant increase in the biomass solubility by 21.9% as chemical oxygen demand and enhanced biogas yield by 157% (567.1 L biogas/kg volatile solids) when the specific energy input was from 200 to 550 kJ/kg. However, only pretreatments where ultrasound was applied at 25–50 kJ/kg led to positive net energy gain, indicating that the biomass processing with this method does not always compensate the energy consumption for irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, biomass constitutes at least 8% of global primary energy supply in Europe [1]. However, the use of renewable energy sources should represent 20% by 2020 and 35% by 2050 [2]. The biomass potential for bioenergy production is affected by biomass supply and demand in various sectors of the economy, especially for the production of food, feed, fiber, and biochemical purposes. In consequence, the increasing attention is now being paid to search the non-food plant species in order to support biofuels production thus reducing the consumption of the food feedstock. The biomass of perennial herbaceous crops e.g., Sida hermaphrodita, Helianthus salicifolius, and Miscanthus × giganteus, offers a promising alternative to conventional energy crops for energy purposes [3]. These plants are characterized by the high biomass yield, valuable biological composition, and low nutrient requirement, and thus can be grown on light soils with low organic matter and nutrient content, unusable for food or feed production [4, 5].
Out of many biological technologies, anaerobic digestion (AD) is the most cost-effective and widely used for commercial production of renewable energy from organic waste biomass [6, 7]. The ratio of energy gain/input in AD was estimated at 28.8 MJ/MJ, which is higher than for other biomass conversion technologies for energy production [8]. However, the direct utilization of lignocellulosic biomass for biogas production is limited because of the complex structure of the plant cell walls. Fermentable sugars of biomass carbohydrates such as cellulose and hemicellulose are trapped inside the cross-linking structure of the lignocellulose which is recalcitrant to biodegradation by enzymes and microbes [7]. Therefore, the pretreatment of biomass is always required to convert lignocellulosic material into a form amenable to biodegradation [9].
The pretreatment step is the most critical challenge for bioenergy production from lignocellulosic agricultural biomass due to the cost efficiency of the bioconversion processes [10]. As a result, the extensive research has been already done on lignocellulosic biomass pretreatment comprised of physical, chemical, and biological methods. As the result, the extensive research has been already done on lignocellulosic biomass pretreatment comprised of physical, chemical, and biological methods. Generally, all pretreatment methods are intended to decrease the crystallinity of cellulose, increase accessible surface area, and reduce lignin content, but not all of them have the positive effects on these factors. Chemical pretreatment methods including acid pretreatment, alkaline pretreatment, and ionic liquids improve the biodegradability of cellulose by delignification of cellulosic material, but they have a lesser impact on depolimerization and decreasing the crystallinity of cellulose complex [11]. The disadvantage of chemical pretreatment is the possible formation of harmful by-products that can adversely affect anaerobic digestion and the cost of chemicals used for treatment [12]. Biological pretreatment is very slow and requires careful control of growth conditions and large amount of space for performing pretreatment [11]. Physical pretreatment methods such as mechanical, hydrothermal, ultrasonic, and microwave radiation pretreatments are effective at reducing the cellulose crystallinity, but they may generate inhibitory by-products [13]. The effects of different pretreatment techniques on the chemical composition and physical characteristics of lignocellulosic biomass have been summarized by Zheng et al. [7]. They stated that the choice of pretreatment method depends on the type of lignocellulosic biomass, and the best method is characterized by improving the digestibility of biomass for AD microbes, avoiding carbohydrate degradation and requiring minimal and inexpensive chemicals and low energy input. Thus, ultrasonic pretreatment is a promising solution due to the low investment and operational convenience [14].
In ultrasonic pretreatment (UP), ultrasound generates monolithic cavitation by passage of ultrasonic waves through the liquid medium, leading to many physical and chemical changes in the liquid solutions [8]. Cavitation is defined as the rapid formation, growth, and subsequent collapse of the bubbles; predominates at low frequencies (< 40 kHz); and causes an intense local heating (around 5000 °C) and high pressure (around 50 Mpa) on a liquid/gas interface, turbulence, and high shearing phenomena in the liquid phase [15,16,17]. The extreme temperatures and pressures generated during cavitational collapse produce highly reactive free radicals (H∙, OH∙, HO2·), which facilitate chemical reactions of organic compounds in the liquid [16]. The cell wall structure of lignocellulosic biomass is disrupted due to the combined effects of both physical and chemical change, resulting in an increase in specific surface area, reducing the degree of polymerization, making cellulose more available to the enzymes through solubilization of hemicelluloses and lignin, eventually leading to the increase in biogas productivity by 4–83% [7, 9, 18, 19].
Considering all of the above, the aim of the study was to determine the effects of ultrasonic pretreatment of the biomass Sida hermaphrodita on fermentative biogas and biomethane production. To the best of our knowledge, there is no study available in the literature focusing on correlating of biogas and methane outputs to composition of lignocellulosic agricultural feedstock and different ultrasonic pretreatment conditions. Thus, a multiple regression model was used to predict energy gain based on experimental data.
Materials and Methods
Substrate Origin and Characteristics
In this study, the silage of Sida hermaphrodita (L.) Rusby mixed with cattle manure was used as a substrate for AD.
Sida hermaphrodita (L.) Rusby was cultivated in the Research and Experimental Station in Łężany of the University of Warmia and Mazury in Olsztyn (Poland), (53°58′00″N, 21°08′27″E). No fertilizers were applied for the plant cultivation. The biomass was harvested in July of the second year of growing by a maize bale chopper, and subsequently ensiled with the bale silage process for 16 weeks.
The cattle manure produced by 360 head of dairy cattle was collected directly from the temporary field storage of solid manure located at the Research Station in Bałdy of the University of Warmia and Mazury in Olsztyn (Poland), (53°36′01″N, 20°36′14″E).
Samples of silage of Sida and cattle manure were collected at five random locations, each in amount of 0.3 kg and then were transported on the same day to the laboratory. The plant material was mixed with cattle manure in weight-based ratio of 2:1 w/w and hydrated with fresh water to obtain 90 ± 0.4% hydratation. Two hundred fifty grams of a mixed sample was homogenized for 20 min (Robo 30, Germany) to obtain a homogeneous mixture with particle size of ± 3.0 mm. The characteristics of a homogenized substrate are shown in Table 1.
Pretreatment Equipment and Procedure
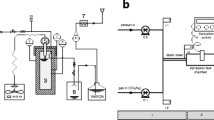
Ultrasonic equipment used in this study was an ultrasonic horn (IS-1, InterSonic, Poland) with a power of 300 W and a frequency of 25 kHz. A volume of 1 L of homogenized sample was manually dosed in the ultrasonic horn. Subsequently, the pretreated sample was mixed with anaerobic inoculum and subjected to respirometric reactors. The experimental design is shown in Fig. 1.
The specific energy input (Es) was calculated as a function of ultrasonic power, the volume of sonicated substrate, and volatile solids (VS) concentration using the following equation:
where P is the applied ultrasonic power in kW, t is the ultrasonic duration in seconds, V is the volume of the substrate, and VS is the volatile solids concentration in kg/L. The setup pretreatment conditions are shown in Table 2. During UP, the increase of temperature created by cavitation was observed; thus, the thermal treatment was also carried out in experimental variants.
The energy output (Eout) generated from biogas production was calculated using the following equation:
where Ybiogas is biogas production in L/gVS, yCH4 is the methane mole fraction in biogas, and ECH4 is the methane heating value in kJ/L. The net energy output (Enout) was calculated by subtracting the energy output in n-variant and the energy output in variant 1 (without pretreatment). The net energy gain (Enet) was calculated by subtracting the net energy output (Enout) and the energy input (Es).
Batch Anaerobic Biodegradability Test
Batch anaerobic biodegradability tests were conducted by using respirometric reactors (WTW GmbH, Germany). A single anaerobic reactor was consisted of the glass bottle with a working volume of 0.5 L coupled tightly with a cap, serving as a pressure sensor to determine the partial pressure increasing induced by biogas production. Pressure in the gaseous phase of the bottle was recorded every 15 min. The respirometric system allowed determining the biogas production based on the pressure changes inside the bottle headspace on the basis of an ideal gas equation. The tests were carried out at a constant temperature of 36 ± 0.5 °C.
At the beginning of the tests, the respirometers were inoculated with 100 mL of anaerobic sludge. The inoculum was collected from agricultural biogas plant operated with liquid manure and crop residues. The main characteristics of the inoculum were total solids (TS) concentration 3.7 ± 0.3%, VS concentration of 69.2 ± 2.8%, and pH 7.57. At the beginning of the tests, anaerobic conditions inside the reactors were met by blowing the bottles with nitrogen gas to remove atmospheric air. In all variants, the initial organic loading rate (OLR) was established on 5.0 g VS/L. Batch anaerobic biodegradability tests were carried out for 40 days. All tests were run in triplicate.
Analytical Methods
The measurements of TS and VS were determined following the gravimetric method. In the solid fraction, the total carbon (TC) and total nitrogen (TN) were determined by elementary particle analyzer Flash 2000 (Thermo Scientific, USA). Soluble fraction (supernatant) for analysis was obtained after centrifugation (5000 rpm, 10 min, MPW-251 Donserv, Poland). Total organic carbon (TOC) was determined in the soluble fraction by a TOC analyzer (TC 100 L, Shimadzu, Japan). Chemical oxygen demand (COD) was determined in the soluble fraction using a DR 5000 spectrophotometer with an HT 200 s mineralizer (Hach-Lange, Germany). The cellulose, hemicellulose, and lignin contents were analyzed in the solid phase of homogenized samples after centrifugation (5000 rpm, 10 min, MPW-251 Donserv, Poland) by ANKOM 220 Fiber Analyzer (ANKOM Technology) with Van Soest method.
Methane content in biogas produced in the gaseous phase of the bottles was measured every 24 h using a gas chromatograph (GC, 7890A Agilent) with a thermal conductivity detector (TCD). The GC was fitted with the two Hayesep Q columns (80/100 mesh), two molecular sieve columns (60/80 mesh), and Porapak Q column (80/100) operating at a temperature of 70 °C. The operational temperatures of the injection and detection ports were set on 150 °C and 250 °C, respectively. Helium and argon were used as the carrier gases at a flow rate of 15 mL/min.
The formulas that can predict biogas and methane outputs depending on Es and the chemical parameters of lignocellulosic agricultural feedstock were developed during the study [20,21,22]. A multiple regression model using a stepwise progressive regression algorithm was used to identify the relevant predictor variables in the formulas, among the investigated variables by Statistica 12.0 PL package (Statsoft, Inc.). Then, the residual analyses were carried out to validate the regression models. The statistical results of the study were analyzed by Statistica 12.0 PL package (Statsoft, Inc.) with a Shapiro-Wilk W-test. One-way analysis of variance (ANOVA) was applied to determine the significance of differences between variables. Variance homogeneity in groups was checked with a Levene’s test, whereas the significance of differences between the analyzed variables was determined with a Tukey RIR test. In all tests, the level of significance was α = 0.05.
Results and Discussion
Effect of UP on Organic Matter Solubilization
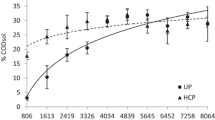
The COD and TOC represent the concentration of soluble organics, and the increase in these concentrations means that UP enhance the transfer of organic matter from lignocellulosic substrate into supernatant. The COD and TOC concentrations before pretreatment were 11,895 mg/L and 3856 mg/L, respectively. Pretreatment of lignocellulosic substrate with ultrasonic irradiation provided the release of COD and TOC from the substrate and solubilization degree increased with the specific energy supplied (Es) (Figs. 2 and 3; Table 3), (p < 0.05). Maximum COD solubilization by 21.9% and TOC solubilization by 24.7% were reached with Es ranging from 200 to 550 kJ/kg. However, it was observed that with the highest specific energy input (Es), the solubilization rate slightly decreased. Pretreatment influenced the characteristics of the mixture of substrate and anaerobic sludge, in which an increase of COD and TOC content by respectively 9.9% and 11.0% with Es ranging between 200 and 550 kJ/kg was observed (Figs. 2 and 3). The high values of the determination coefficient (R2) demonstrated a good fitting of the experimental data to the model proposed (Figs. 2 and 3). The cellulose, hemicellulose, and lignin concentrations in the solid phase of the raw substrate were decreased with an increase in the specific energy input (Figs. 4, 5, and 6), which means that they have been dissolved. The maximum decrease in the concentration of cellulose was significantly higher than the observed decreases in hemicellulose and lignin, whose values were by 33.6%, 27.2%, and 14.0%, respectively (p < 0.05).
Taking into account other works [23,24,25], it could be assumed that ultrasonic pretreatment enhances delignification and solubilization of the plant materials. In a liquid medium, ultrasonication breaks the bonds between lignin monomers to breakdown the lignin structure [9, 23, 26]. The cavitation process takes place most actively at the liquid-solid interphase, leading to contracting, expanding, and finally fractionating of the lignocellulosic biomass [27, 28]. The partial decomposition of lignin facilitates enzymes to access the glycoside bonds of polysaccharides, leading to increasing bioconversion of the plant substrate [29]. Garcia et al. [30] observed decreasing contents of hemicellulose in the solid samples of olive tree pruning residues treated with ultrasounds over 30 min and increasing of hemicelluloses and lignin in the liquid fraction. According to the literature, sonication enhanced degradation of lignin by 10%; however, it did not affect the fibrillary structure of cellulose [24, 25, 30, 31]. Sul’man et al. [28] demonstrated the effects of UP (368 W/cm2, 30 kHz) on cellulose and lignin degradation of sunflower husk biomass when the treatment time was no longer than 15 min. After that time, their contents increased gradually, which was associated with the initiation of the anew lignin polymerization process. In our study, we also observed the increasing concentration of lignin in the solid phase of the substrate when the irradiation time was higher than 16 min. Generally, higher energy inputs lead to the formation of large biomass particles due to re-flocculation process; in contrast, low energy inputs and short pretreatment time only enhance biomass deagglomeration instead of its solubilization [32, 33]. An increase in the intensity of ultrasound enhanced the polysaccharide extraction from lignocellulosic biomass and enhanced the degradation of compounds with high molecular weight, e.g., lignin [28]. According to Rehman et al. [34], the efficiency of ultrasonic pretreatment increases with energy input increasing until the increasing number of cavitational bubbles is as much that starts to decrease energy transfer to the liquid. In our study, the optimum Es for pretreatment of the mixture of Sida hermaphrodita and cattle manure at which the most complete destruction of the substrate was achieved ranged from 200 to 350 kJ/kg.
Effect of UP on Biogas Production
Figure 7 illustrates the experimental results of the batch anaerobic biodegradability tests. The statistical analyses allowed the calculation of the determination coefficient (R2), which was higher than 0.95 in estimation of the methane yield and as low as 0.87 in biogas yield analysis. The raw substrate without pretreatment showed the biogas and methane yields of 341.4 L/kg VS and 139.8 L/kg VS, respectively. Ultrasonic pretreatment had significant effect on the biogas yield (p < 0.05) when the specific energy input (Es) ranges between 100 and 550 kJ/kg. Lower energy inputs had no significant effects on biogas yield. The highest biogas production of 567.1 L/kg VS was achieved with the highest specific energy input; however, there was no difference when Es ranges from 100 to 550 kJ/kg. In turn, sonication of the raw substrate had significant effect on methane yield under all pretreatment conditions (p < 0.05). The highest methane yields ranged between 318.0 and 337.9 L/kg VS with Es of 200–550 kJ/kg (p > 0.05).
Significant improvement in methane yield by ultrasound was observed by several researchers [18, 33, 35]. According to Schroyen et al. [36], the methane production from lignocellulosic crops was in the range of 170 to 390 L/kg VS and substrate composition, especially the lignin content, highly affected the results of methane production [37, 38]. Under the studied conditions, maximum methane yield was lower than the maximum values for lignocellulosic materials. However, ultrasonic pretreatment enhanced biogas evolution by 142%. Similarly, Castrillón et al. [33] observed increase in biogas production from cattle manure pretreated by ultrasound by 121%. Pretreatment of waste activated sludge by ultrasound enhanced the biogas production by 172.56% [39]. In turn, Pérez-Rodríguez et al. [24] observed that the sonication of corn cob did not allow enhancing the methane yield.
Methane concentration in biogas increased due to pretreatment, and the increase was found to be significant with respect to the level achieved in the substrate without UP (Fig. 8). Maximum methane concentration of 59.6% was noted with the highest specific energy input; however, there was no difference in methane content in biogas (p > 0.05) when ultrasonic energy input ranges from 200 to 350 kJ/kg. It is in contrast to the findings of other researchers, who did not observe the increase in methane concentration in biogas due to UP [18, 33, 40].
The mathematical formula of biogas yield (3) from lignocellulosic agricultural feedstock is characterized by a standard error of ± 12.36 and represent about 95.84% of changes observed in the biogas production process (coefficient of determination R2 = 0.9584). The formula of methane yield (4) is characterized by a standard error of ± 10.63 and represent about 96.45% of changes observed in the process (coefficient of determination R2 = 0.9645).
where B is the predicted yield of biogas in L/kg VS, M is the predicted yield of methane in L/kg VS, Es is the specific energy input of ultrasonication in kJ/kg, C is the cellulose content in the solid fraction of lignocellulosic biomass in mg/L, H is the hemicellulose content in the solid fraction of lignocellulosic biomass in mg/L, L is the lignin content in the solid fraction of lignocellulosic biomass in mg/L, and TOC is the total organic carbon concentration in the soluble fraction of lignocellulosic biomass in mg/L.
The effectiveness of ultrasonic pretreatment of agricultural biomass is strongly depending on the feedstock characteristics, and these formulas allow for basic prediction of biogas and methane yield from lignocellulosic biomass pretreated with ultrasound and does not require a large number of predictor data.
Effect of UP on Anaerobic Digestate Characteristic
In our study, the reduction in TOC, COD, and VS (as % TS) was observed (Figs. 2, 3, and 9) which was the result of biogas production. When ultrasonic pretreatment was used, the TOC reduction ranged from 71.7% (Es of 25 kJ/kg) to 76.6% (Es of 550 kJ/kg) (Fig. 2). For the raw substrate, the TOC reduction achieved 62.8% (p < 0.05). The COD reduction ranged from 70.9 to 76.9% when the substrate was pretreated with ultrasound and 68.5% without pretreatment (p < 0.05%), (Fig. 3). They corresponded to VS reduction, which was the highest with ultrasonic energy input of 550 kJ/kg (Fig. 9). However, no significant differences in VS reduction were observed in samples pretreated with ultrasound and without pretreatment (p > 0.05). Zeynali et al. [35] noted significant effects of ultrasounds on TS and VS reduction in fruit and vegetable wholesale market waste samples. They obtained 63% of VS and 58% of TS reductions in sonicated samples, which were respectively 8% and 12% higher than in samples without pretreatment.
Energy Balance of UP
The energy gain assessment of anaerobic digestion of lignocellulosic agricultural feedstock pretreated with ultrasonic is presented in Table 4. The net energy output gained from methane production was increased with the specific energy input increasing, because UP enhanced the biogas yield. However, only ultrasonic pretreatments with Es between 25 and 50 kJ/kg led to the positive net energy gain. In other variants (from 4 to 7), the negative energy balances were noted because the amount of energy inputs (Es) used for irradiation of the substrate far exceeded the amount of energy produced from biogas. It could be states that from economic point of view, ultrasonic pretreatment is not suitable for lignocellulosic agricultural biomass pretreatment.
Conclusions
Ultrasonication of Sida hermaphrodita mixed with cattle manure resulted in significant effects on organic matter solubilization by 21.9% as COD and by 24.7% as TOC with the specific energy input ranged from 200 to 550 kJ/kg. The maximum decrease in the concentration of cellulose, hemicellulose, and lignin in the solid phase of the raw substrate was 33.6%, 27.2%, and 14.0%, respectively. Significant improvement in biogas/methane yield by ultrasonic pretreatment was observed. The methane yields ranging between 318.0 and 337.9 L/kg VS with Es of 200–550 kJ/kg were achieved. Ultrasonic pretreatment increased the net energy output gained from methane production. However, the overall process results were energetically favorable only when Es ranged from 25 to 50 kJ/kg, which indicated that the increased methane yield by UP did not compensate the energy consumption for irradiation.
References
Faaij APC (2018) Securing sustainable resource availability of biomass for energy applications in Europe, review of recent literature. http://bioenergyeurope.org/wp-content/uploads/2018/11/Bioenergy-Europe-EU-Biomass-Resources-Andr%C3%A9-Faaij-Final.pdf
Renewable Energy Directive (RED) 2009/EC/28 EC (2009)
Stolarski MJ, Krzyżaniak M, Warmiński K, Tworkowski J, Szczukowski JS, Olba-Zięty E, Gołaszewski J (2017) Energy efficiency of perennial herbaceous crops production depending on the type of digestate and mineral fertilizers. Energy Educ Sci Tech Part A: Energy Sci Res 134:50–60
Nabel M, Temperton VM, Poorter H, Lücke A, Jablonowski ND (2016) Energizing marginal soils – the establishment of the energy crop Sida hermaphrodita as dependent on digestate fertilization, NPK, and legume intercropping. Biomass Bioenergy 87:9–16
Oleszek M, Matyka M, Lalak J, Tys J, Paprota E (2013) Characterization of Sida hermaphrodita as a feedstock for anaerobic digestion process. J Food Agric Environ 11:1839–1841
Bharathiraja B, Sudharsana T, Jayamuthunagai J, Praveenkumar R, Chozhavendhan S, Iyyappan J (2018) Biogas production – a review on composition, fuel properties, feed stock and principles of anaerobic digestion. Renew Sust Energ Rev 90:570–582
Zheng Y, Zhao J, Xu F, Li Y (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog Energy Combust Sci 42:35–53
Kumari D, Singh R (2018) Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renew Sust Energ Rev 90:877–891
You Z, Pan S-Y, Sun N, Kim H, Chiang P-C (2019) Enhanced corn stover fermentation for biogas production by NaOH pretreatment with CaO additive and ultrasound. J Clean Prod 238:117813
Zheng Y, Shi J, Tu M, Cheng YS (2017) Principles and development of lignocellulosic biomass pretreatment for biofuels (chapter one). Adv Bioenergy 2:1–68
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci 38:522–555
Cesaro A, Belgiorno V (2014) Pretreatment methods to improve anaerobic biodegradability of organic municipal solid waste fractions. Chem Eng J 240:24–37
Amin FR, Khalid H, Zhang H, Rahman S, Zhang R, Liu G, Chen C (2017) Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 7:72
Zou S, Wang X, Chen Y, Wan H, Feng Y (2016) Enhancement of biogas production in anaerobic co-digestion by ultrasonic pretreatment. Energy Convers Manag 112:226–235
Avvaru B, Venkateswaran N, Uppara P, Iyengar SB, Katti SS (2018) Current knowledge and potential applications of cavitation technologies for the petroleum industry. Ultrason Sonochem 42:493–507
Harris PW, McCabe BK (2015) Review of pre-treatments used in anaerobic digestion and their potential application in high-fat cattle slaughterhouse wastewater. Appl Energy 155:560–575
Carrère H, Dumas C, Battimelli A, Batstone DJ, Delgenès JP, Steyer JP, Ferrer I (2010) Pretreatment methods to improve sludge anaerobic degradability: a review. J Hazard Mater 183:1–15
Neumann P, González Z, Vidal G (2017) Sequential ultrasound and low-temperature thermal pretreatment: process optimization and influence on sewage sludge solubilization, enzyme activity and anaerobic digestion. Bioresour Technol 234:178–187
Carrere H, Antonopoulou G, Affes R, Passos F, Battimelli A, Lyberatos G, Ferrer I (2016) Review of feedstock pretreatment strategies for improved anaerobic digestion: from lab-scale research to full-scale application. Bioresour Technol 199:386–397
Fumo N, Rafe BMA (2015) Regression analysis for prediction of residential energy consumption. Renew Sust Energ Rev 47:332–343
Silhavy R, Silhavy P, Zdenka P (2017) Analysis and selection of a regression model for the use case points method using a stepwise approach. J Syst Softw 125:1–14
Kokaly RF, Clark RN (1999) Spectroscopic determination of leaf biochemistry using band-depth analysis of absorption features and stepwise multiple linear regression. Remote Sens Environ 67:267–287
Hassan S, Williams GA, Jaiswal AK (2018) Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour Technol 262:310–318
Pérez-Rodríguez N, García-Bernet D, Domínguez JM (2016) Effects of enzymatic hydrolysis and ultrasounds pretreatments on corn cob and vine trimming shoots for biogas production. Bioresour Technol 221:130–138
Iskalieva A, Yimmou BM, Gogate PR, Horvath M, Horvath PG, Csoka L (2012) Cavitation assisted delignification of wheat straw: a review. Ultrason Sonochem 19:984–993
Tian SQ, Wang ZY, Fan ZL, Zuo LL (2012) Comparison of ultrasonic and CO2 laser pretreatment methods on enzyme digestibility of corn stover. Int J Mol Sci 13:4141–4152
Velmurugan R, Muthukumar K (2012) Sono-assisted enzymatic saccharification of sugar cane bagasse for bioethanol production. Biochem Eng J 63:1–9
Sul’man EM, Sul’man MG, Prutenskaya EA (2011) Effect of ultrasonic pretreatment on the composition of lignocellulosic material in biotechnological processes. Catal Ind 3:28–33
Madison MJ, Coward-Kelly G, Liang C, Karim MN, Falls M, Holtzapple T (2017) Mechanical pretreatment of biomass – part I: acoustic and hydrodynamic cavitation. Biomass Bioenergy 98:135–141
García A, González Alriols M, Llano-Ponte R, Labidi J (2011) Ultrasound-assisted fractionation of the lignocellulosic. Bioresour Technol 102:6326–6330
Yu J, Zhang J, He J, Liu Z, Yu Z (2009) Combinations of mild physical or chemical pretreatment with biological pretreatment for enzymatic hydrolysis of rice hull. Bioresour Technol 100:903–908
Quiroga G, Castrillón L, Fernández-Nava Y, Marañón E, Negral L, Rodríguez-Iglesias J, Ormaechea P (2014) Effect of ultrasound pre-treatment in the anaerobic co-digestion of cattle manure with food waste and sludge. Bioresour Technol 154:74–79
Castrillón L, Fernández-Nava Y, Ormaechea P, Marañón E (2011) Optimization of biogas production from cattle manure by pre-treatment with ultrasound and co-digestion with crude glycerin. Bioresour Technol 102:7845–7849
Rehman MSU, Kim I, Chisti Y, Han JI (2013) Use of ultrasound in the production of bioethanol from lignocellulosic biomass. Energy Educ Sci Tech Part A: Energy Sci Res 30:1391–1410
Zeynali R, Khojastehpour M, Ebrahimi-Nik M (2017) Effect of ultrasonic pre-treatment on biogas yield and specific energy in anaerobic digestion of fruit and vegetable wholesale market wastes. Sustain Environ Res 27:259–264
Schroyen M, Vervaeren H, Van Hulle SWH, Raes K (2014) Impact of enzymatic pretreatment on corn stover degradationand biogas production. Bioresour Technol 173:59–66
Menardo S, Airoldi G, Cacciatore V, Balsari P (2015) Potential biogas and methane yield of maize stover fractions and evaluation of some possible stover harvest chains. Bioresour Technol 129:352–359
Dandikas V, Heuwinkel H, Lichti F, Drewes JE, Koch K (2014) Correlation between biogas yield and chemical composition of energy crops. Bioresour Technol 174:316–320
Zhang Z, Zhang L, Zhou Y, Chen J, Liang Y, Wei L (2013) Pilot-scale operation of enhanced anaerobic digestion of nutrient-deficient municipal sludge by ultrasonic pretreatment and co-digestion of kitchen garbage. J Environ Chem Eng 1:73–78
Bougrier C, Albasi C, Delgenès JP, Carrère H (2006) Effect of ultrasonic, thermal and ozone pre-treatments on waste activated sludge solubilisation and anaerobic biodegradability. Chem Eng Process 45:711–718
Funding
The work was supported by the National Centre for Research and Development, Poland [grant number BIOSTRATEG 1/270745/2/NCBR/2015] entitled “Dietary, power, and economic potential of Sida hermaphrodita cultivation on fallow land” (acronym SIDA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marta, K., Paulina, R., Magda, D. et al. Evaluation of Ultrasound Pretreatment for Enhanced Anaerobic Digestion of Sida hermaphrodita. Bioenerg. Res. 13, 824–832 (2020). https://doi.org/10.1007/s12155-020-10108-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10108-9