Abstract
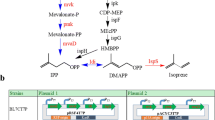
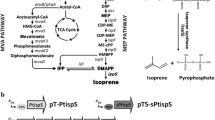
There is a need to develop renewable fuels and chemicals that will help meet global demands for energy and synthetic chemistry feedstock, without contributing to climate change or environmental degradation. Isoprene (C5H8) is one such key chemical ingredient, required for the production of synthetic rubber or plastic products, and a potential biofuel. Enabling a sustainable microbial fermentation for the production of isoprene is an attractive alternative to a petroleum origin. This work demonstrates transgenic expression of the Pueraria montana (kudzu vine) isoprene synthase gene (kIspS) and heterologous isoprene production in Escherichia coli. Enhancements in the amount of E. coli isoprene production were achieved upon over-expression of the native 2-C-methyl-d-erythritol-4-phosphate (MEP) biosynthetic pathway and, independently, upon heterologous over-expression of the entire mevalonic acid (MVA) pathway. A direct comparison of the efficiency of cellular organic carbon flux through the MEP and MVA pathways is provided, under conditions when these are expressed in the same host using the same plasmid, and same ribosome-binding sites (RBS). These alternative isoprenoid biosynthetic pathways were assembled in and expressed through a superoperon, suitable for transformation of E. coli. Introduction of specific RBS and nucleotide spacers between individual genes in the superoperon structure enabled maximal expression in E. coli batch cultures and translated to an improved production from 0.4 mg isoprene per liter of culture (control) to 5 mg isoprene per liter of culture (MEP superoperon transformants) and up to 320 mg isoprene per liter of culture (MVA superoperon transformants). This 800-fold increase in isoprene concentration from the MVA transformants and the attendant isoprene-to-biomass 0.78:1 carbon partitioning ratio suggested that the engineered MVA pathway introduces a bypass in the flux of endogenous substrate in E. coli to isopentenyl-diphosphate and dimethylallyl-diphosphate, thus overcoming flux limitations imposed upon the regulation of the native MEP pathway by the cell.










Similar content being viewed by others
Abbreviations
- IPP:
-
Isopentenyl-diphosphate
- DMAPP:
-
Dimethylallyl-diphosphate
- DCW:
-
Dry cell weight
- RBS:
-
Ribosome-binding site
- TIR:
-
Translation initiation region
References
Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T et al (1995) A global model of natural volatile organic compound emissions. J Geophys Res 100:8873–8892
Sharkey TD, Singsass EL (1995) Why plants emit isoprene? Nature 374:769–769
Paulot F, Crounse JD, Kjaergaard HG, Kürten A, St Clair JM, Seinfeld JH et al (2009) Unexpected epoxide formation in the gasphase photooxidation of isoprene. Science 325:730–733
Behnke K, Ehlting B, Teuber M, Bauerfeind M, Louis S, Hänsch R et al (2007) Transgenic, nonisoprene emitting poplars don’t like it hot. Plant J 51:485–499
Singsaas EL, Lerdau M, Winter K, Sharkey TD (1997) Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol 115:1413–1420
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Vickers CE, Possell M, Cojocariu CI, Velikova VB, Laothawornkitkul J, Ryan A et al (2009) Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant Cell Environ 32:520–531
Loivamäki M, Mumm R, Dicke M, Schnitzler JP (2008) Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proc Natl Acad Sci U S A 105:17430–17435
Laothawornkitkul J, Paul ND, Vickers CE, Possell M, Taylor JE, Mullineaux PM et al (2008) Isoprene emissions influence herbivore feeding decisions. Plant Cell Environ 31:1410–1415
Miller B, Oschinski C, Zimmer W (2001) First isolation of an isoprene synthase gene from poplar and successful expression of the gene in Escherichia coli. Planta 213:483–487
Silver GM, Fall R (1995) Characterization of aspen isoprene synthase, an enzyme responsible for leaf isoprene emission to the atmosphere. J Biol Chem 270:13010–13016
Fortunati A, Barta C, Brilli F, Centritto M, Zimmer I, Schnitzler JP et al (2008) Isoprene emission is not temperature-dependent during and after severe drought-stress: a physiological and biochemical analysis. Plant J 55:687–697
Sasaki K, Ohara K, Yazaki K (2005) Gene expression and characterization of isoprene synthase from Populus alba. FEBS Lett 579:2514–2518
Sharkey TD, Yeh S, Wiberley AE, Falbel TG, Gong D, Fernandez DE (2005) Evolution of the isoprene biosynthetic pathway in kudzu. Plant Physiol 137:700–712
Köksal M, Zimmer I, Schnitzler JP, Christianson DW (2010) Structure of isoprene synthase illuminates the chemical mechanism of teragram atmospheric carbon emission. J Mol Biol 402(2):363–373
Lindberg P, Park S, Melis A (2010) Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab Eng 12:70–79
Bentley FK, Melis A (2012) Diffusion-based process for carbon dioxide uptake and isoprene emission in gaseous/aqueous two-phase photobioreactors by photosynthetic microorganisms. Biotech Bioeng 109:100–109
Whited GM, Feher FJ, Benko DA, Cervin MA, Chotani GK, McAuliffe JC et al (2010) Development of a gas-phase bioprocess for isoprene-monomer production using metabolic pathway engineering. Ind Biotechnol 6(3):152–163
Zhao Y, Yang J, Qin B, Li Y, Sun Y, Su S et al (2011) Biosynthesis of isoprene in Escherichia coli via methylerythritol phosphate (MEP) pathway. Appl Microbiol Biotechnol 90(6):1915–1922
Leonard E, Ajikumar PK, Thayer K, Xiao WH, Mo JD, Tidor B et al (2010) Combining metabolic and protein engineering of a terpenoid biosynthetic pathway for overproduction and selectivity control. Proc Natl Acad Sci U S A 107(31):13654–13659
Martin VJ, Pitera DJ, Withers ST, Newman JD, Keasling JD (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol 21(7):796–802
Lange BM, Rujan T, Martin W, Croteau R (2000) Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc Natl Acad Sci U S A 97(24):13172–13177
Lee PC, Schmidt-Dannert C (2002) Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl Microbiol Biotechnol 60(1–2):1–11
Lichtenthaler HK (2000) Non-mevalonate isoprenoid biosynthesis: enzymes, genes and inhibitors. Biochem Soc Trans 28:785–789
Rohmer M (1999) The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep 16(5):565–574
Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H (1996) Glyceraldehyde3-phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis. J Am Chem Soc 118:2564–2566
Farmer WR, Liao JC (2001) Precursor balancing for metabolic engineering of lycopene production in Escherichia coli. Biotechnol Prog 17(1):57–61
Kajiwara S, Fraser PD, Kondo K, Misawa N (1997) Expression of an exogenous isopentenyl diphosphate isomerase gene enhances isoprenoid biosynthesis in Escherichia coli. Biochem J 324:421–426
Kim SW, Keasling JD (2001) Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol Bioeng 72(4):408–415
Vadali RV, Fu Y, Bennett GN, San KY (2005) Enhanced lycopene productivity by manipulation of carbon flow to isopentenyl diphosphate in Escherichia coli. Biotechnol Prog 21(5):1558–1561
Yoon SH, Lee SH, Das A, Ryu HK, Jang HJ, Kim JY et al (2009) Combinatorial expression of bacterial whole mevalonate pathway for the production of beta-carotene in E. coli. J Biotechnol 140(3–4):218–226
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, pp 11–730
Russell RG (2007) Bisphosphonates: mode of action and pharmacology. Pediatrics 119(Suppl 2):S150–S162
Melis A (2011) Short chain volatile hydrocarbon production using genetically engineered microalgae, cyanobacteria or bacteria. United States Patent 7,947,478
Cunningham FX Jr, Sun Z, Chamovitz D, Hirschberg J, Gantt E (1994) Molecular structure and enzymatic function of lycopene cyclase from the cyanobacterium Synechococcus sp strain PCC7942. Plant Cell 6(8):1107–1121
Williams DC, McGarvey DJ, Katahira EJ, Croteau R (1998) Truncation of limonene synthase preprotein provides a fully active ‘pseudomature’ form of this monoterpene cyclase and reveals the function of the amino-terminal arginine pair. Biochemistry 37(35):12213–12220
Ringquist S, Shinedling S, Barrick D, Green L, Binkley J, Stormo GD et al (1992) Translation initiation in Escherichia coli: sequences within the ribosome-binding site. Mol Microbiol 6(9):1219–1229
Lehning A, Zimmer I, Steinbrecher R, Brüggemann N, Schnitzler J-P (1999) Isoprene synthase activity and its relation to isoprene emission in Quercus robur L leaves. Plant Cell Environ 22:495–504
Schnitzler J-P, Arenz R, Steinbrecher R, Lehning A (1996) Characterization of an isoprene synthase from leaves of Quercus petraea (Mattuschka) Liebl. Bot Acta 109:216–221
Yazdani SS, Gonzalez R (2007) Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr Opin Biotechnol 18(3):213–219
Dasari MA, Kiatsimkul PP, Sutterlin WR, Suppes GJ (2005) Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl Catal Gen 281:225–231
Salis H, Mirsky EA, Voigt CA (2009) Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol 27:946–950. doi:dx.doi.org
Luria SE (1960) The bacterial protoplasm: composition and organization. In: Gunsales IC, Stanier RY (eds) The bacteria, vol 1. Academic, New York, pp 1–34
Acknowledgments
A. Z. is the recipient of a Swiss National Science Foundation Postdoctoral Fellowship, grant no. PBBEP3_128360.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zurbriggen, A., Kirst, H. & Melis, A. Isoprene Production Via the Mevalonic Acid Pathway in Escherichia coli (Bacteria). Bioenerg. Res. 5, 814–828 (2012). https://doi.org/10.1007/s12155-012-9192-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-012-9192-4