Abstract
Objective
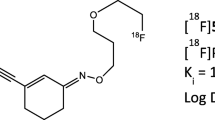
5-(1-(2-[18F]fluoroethoxy))-[3-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-propyl]-5,6,7,8-tetrahydronaphthalen ([18F]MC225) is a selective substrate for P-glycoprotein (P-gp), possessing suitable properties for measuring overexpression of P-gp in the brain. This is the first-in-human study to examine safety, radiation dosimetry and P-gp function at the blood–brain barrier (BBB) of [18F]MC225 in healthy subjects.
Methods
[18F]MC225 biodistribution and dosimetry were determined in 3 healthy male subjects, using serial 2 h and intermittent 4 and 6 h whole-body PET scans acquired after [18F]MC225 injection. Dynamic [18F]MC225 brain PET (90 min) was obtained in 5 healthy male subjects. Arterial blood was sampled at various time intervals during scanning and the fraction of unchanged [18F]MC225 in plasma was determined. T1-weighted MRI was performed for anatomical coregistration. Total distribution volume (VT) was estimated using 1- and 2-tissue-compartment models (1-TCM and 2-TCM, respectively). VT was also estimated using the Logan graphical method (Logan plot) (t* = 20 min). Surrogate parameters without blood sampling (area-under the curve [AUC] of regional time–activity curves [TACs] and negative slope of calculated TACs) were compared with the VT values.
Results
No serious adverse events occurred throughout the study period. Although biodistribution implied hepatobiliary excretion, secretion of radioactivity from liver to small intestine through the gallbladder was very slow. Total renal excreted radioactivity recovered during 6 h after injection was < 2%ID. Absorbed dose was the highest in the pancreas (mean ± SD, 203 ± 45 μGy/MBq) followed by the liver (83 ± 11 μGy/MBq). Mean effective dose with and without urination was 17 ± 1 μSv/MBq. [18F]MC225 readily entered the brain, distributing homogeneously in grey matter regions. 2-TCM provided lower Akaike information criterion scores than did 1-TCM. VT estimated by Logan plot was well correlated with that of 2-TCM (r2 > 0.9). AUCs of TACs were positively correlated with VT (2-TCM) values (r2: AUC0-60 min = 0.61, AUC0-30 min = 0.62, AUC30-60 min = 0.59, p < 0.0001). Negative slope of SUV TACs was negatively correlated with VT (2-TCM) values (r2 = 0.53, p < 0.0001).
Conclusions
This initial evaluation indicated that [18F]MC225 is a suitable and safe PET tracer for measuring P-gp function at the BBB.







Similar content being viewed by others
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Terasaki T, Ohtsuki S. Brain-to-blood transporters for endogenous substrates and xenobiotics at the blood-brain barrier: an overview of biology, and methodology. NeuroRx. 2005;2:63–72.
Giacomini KM, Huang S-M. Transporters in drug development and clinical pharmacology. Clin Pharmacol Ther. 2013;94:3–9.
Graff CL, Pollack GM. Drug transporter at the blood–brain barrier and the choroid plexus. Curr Drug Metab. 2004;5:95–108.
Löscher W, Potschka H. Drug resistance in brain disease and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602.
Feldmann M, Koepp M. ABC transporter and drug resistance in patients with epilepsy. Curr Pharm Des. 2016;22:5793–807.
Ilyas-Feldmann M, Asselin M-C, Wang S, McMahon A, Anton-Rodriguez A, Brown G, et al. P-glycoprotein overactivity in epileptogenic developmental lesions measured in vivo using (R)-[11C]verapamil PET. Epilepsia. 2020;61:1472–80.
Volk HA, Löscher W. Multidrug resistance in epilepsy: rats with drug-resistant seizures exhibit enhanced brain expression of P-glycoprotein compared with rats with drug-responsive seizures. Brain. 2005;128:1358–68.
O’Brien FE, Clarke G, Fitzgerald P, Dinan TG, Griffin BT, Cryan JF. Inhibition of P-glycoprotein enhances transport of imipramine across the blood-brain barrier: microdialysis studies in conscious freely moving rats. Br J Pharmacol. 2012;166:1333–43.
de Klerk OL, Willemsen AT, Roosink M, Bartels AL, Hendrikse NH, Bosker FJ, et al. Locally increased P-glycoprotein function in major depression: a PET study with [11C]verapamil as a probe for P-glycoprotein function in the blood–brain barrier. Int J Neuropsychopharmacol. 2009;12:895–904.
Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, et al. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-protease inhibitors. J Clin Invest. 1998;101:289–94.
Chai AB, Leung GKF, Callaghan R, Gelissen IC. P-glycoprotein: a role in the export of amyloid-β in Alzheimer’s disease? FEBS J. 2020;287:612–25.
Deo AK, Borson S, Link JM, Domino K, Eary JF, Ke B, et al. Activity of p-glycoprotein, a β-amyloid transporter at the blood–brain barrier, is compromised in patients with mild Alzheimer disease. J Nucl Med. 2014;55:1106–11.
van Assema DME, Lubberink M, Bauer M, van der Filer WM, Schuit RC, Windhorst AD, et al. Blood–brain barrier P-glycoprotein function in Alzheimer’s disease. Brain. 2012;135:181–9.
Bartels AL, Willemsen ATM, Kortekaas R, de Jong BM, de Vries R, de Klerk O, et al. Decreased blood–brain barrier P-glycoprotein function in the progression of Parkinson’s disease PSP and MSA. J Neural Transm (Vienna). 2008;115:1001–9.
Vautier S, Fernandez C. ABCB1: the role in Parkinson’s disease and pharmacokinetics of antiparkinsonian drugs. Expert Opin Drug Metab Toxicol. 2009;5:1349–58.
Luurtsema G, Elsinga P, Dierckx R, Boellaard R, van Waarde A. PET tracers for imaging of ABC transporters at the blood–brain barrier: principles and strategies. Curr Pharm Des. 2016;22:5779–85.
Raaphorst RM, Windhorst AD, Elsinga PH, Colabufo NA, Lammertsma AA, Luurtsema G. Radiopharmaceuticals for assessing ABC transporters at the blood–brain barrier. Clin Pharmacol Ther. 2015;97:362–71.
Lubberink M. Kinetic models for measuring P-glycoprotein function at the blood-brain barrier with positron emission tomography. Curr Pharm Des. 2016;22:5786–92.
Luurtsema G, Molthoff CFM, Schuit RC, Windhorst AD, Lammertsma AA, Franssen EJF. Evaluation of (R)-[11C]verapamil as PET tracer of P-glycoprotein function in the blood–brain barrier: kinetics and metabolism in the rat. Nucl Med Biol. 2005;32:87–93.
Savolainen H, Windhorst AD, Elsinga PH, Cantore M, Colabufo NA, Willemsen AT, et al. Evaluation of [18F]MC225 as a PET radiotracer for measuring P-glycoprotein function at the blood–brain barrier in rats: kinetics, metabolism, and selectivity. J Cereb Blood Flow Metab. 2017;37:1286–98.
Savolainen H, Cantore M, Colabufo NA, Elsinga PH, Windhorst AD, Luurtsema G. Synthesis and preclinical evaluation of three novel fluorine-18 labeled radiopharmaceuticals for P-glycoprotein PET imaging at the blood–brain barrier. Mol Pharm. 2015;12:2265–75.
García-Varela L, Arif WM, García DV, Kakiuchi T, Ohba H, Harada N, et al. Pharmacokinetic modeling of [18F]MC225 for quantification of the P-glycoprotein function at the blood–brain barrier in non-human primates with PET. Mol Pharm. 2020;17:3477–86.
García-Varela L, García DV, Aguiar P, Kakiuchi T, Ohba H, Harada N, et al. Head-to-head comparison of (R)-[11C]verapamil and [18F]MC225 in non-human primates, tracers for measuring P-glycoprotein function. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05411-2.
Toyohara J, Sakata M, Tago T, Colabufo NA, Luurtsema G. Automated synthesis, preclinical toxicity, and radiation dosimetry of [18F]MC225 for clinical use: a tracer for measuring P-glycoprotein function at the blood–brain barrier. EJNMMI Res. 2020;10:84.
Zanotti-Fregonara P, Lammertsma AA, Innis RB. Suggested pathway to assess radiation safety of 18F-labeled PET tracers for first-in-human studies. Eur J Nucl Med Mol Imaging. 2013;40:1781–3.
Toyohara J, Sakata M, Oda K, Ishii K, Ito K, Hiura M, et al. Initial human PET studies of metabotropic glutamate receptor type 1 ligand 11C-ITMM. J Nucl Med. 2013;54:1302–7.
Ito K, Sakata M, Oda K, Wagatsuma K, Toyohara J, Ishibashi K, et al. Comparison of dosimetry between PET/CT and PET alone using 11C-ITMM. Austr Phys Eng Sci Med. 2016;39:177–86.
Wagatsuma K, Miwa K, Sakata M, Oda K, Ono H, Kameyama M, et al. Comparison between new-generation SiPM-based and conventional PMT-based TOF-PET/CT. Phys Med. 2017;42:203–10.
Kirschner AS, Ice RD, Beierwaltes WH. Radiation dosimetry of 131I–19-iodo-cholesterol: the pitfalls of using tissue concentration data reply. J Nucl Med. 1975;16:248–9.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7.
ICRP. The 2007 recommendations of the international commission on radiological protection ICRP publication 103. Ann ICRP. 2007;37:1–332.
Toyohara J, Yamamoto H, Tago T. Searching for diagnostic properties of novel fluorine-18-labeled D-allose. Ann Nucl Med. 2019;33:855–65.
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time–activity measurement applied to [N-11C-methyl]-(–)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–7.
Auvity S, Caillé F, Marie S, Wimberley C, Bauer M, Langer O, et al. P-Glycoprotein (ABCB1) inhibits the influx and increases the efflux of 11C-metoclopramide across the blood–brain barrier: a PET study on nonhuman primates. J Nucl Med. 2018;59:1609–15.
Tournier N, Bauer M, Picher V, Nics L, Klebermass E-M, Bamminger K, et al. Impact of P-glycoprotein function on the brain kinetics of the weak substrate 11C-metoclopramide assessed with PET imaging in humans. J Nucl Med. 2019;60:985–91.
Fusi F, Durante M, Gorelli B, Perrone MG, Colabufo NA, Saponara S. MC225, a novel probe for P-glycoprotein PET imaging at the blood–brain barrier: in vitro cardiovascular safety evaluation. J Cardiovasc Pharmacol. 2017;70:405–10.
Brzezińska E. Synthesis and pharmacological properties of 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline derivatives. Acta Pol Pharm. 1996;53:365–71.
ICRP. Radiological protection in biomedical research. ICRP Publication 62. Ann ICRP. 1992;22:1–18.
RDRC Final Guidance: human research without an investigational new drug application. 2010 https://www.fda.gov/media/76286/download. (Accessed Aug 2010).
Staud F, Ceckova M, Micuda S, Pavek P. Expression and function of P-glycoprotein in normal tissues: effect on pharmacokinetics. Methods Mol Biol. 2010;596:199–222.
Bartels A, Kortekaas R, Bart J, Willemsen ATM, de Klerk OL, de Vries JJ, et al. Blood–brain barrier P-glycoprotein function decreases in specific brain regions with aging: a possible role in progressive neurodegeneration. Neurobiol Aging. 2009;30:1818–24.
Bauer M, Karch R, Neumann F, Abrahim A, Wagner CC, Kletter K, et al. Age dependency of cerebral P-gp function measured with (R)-[11C]verapamil PET. Eur J Clin Pharmacol. 2009;65:941–6.
Chiu C, Miller MC, Monahan R, Osgood DP, Stopa EG, Silverberg GD. P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: preliminary observations. Neurobiol Aging. 2015;36:2475–82.
Toornvliet R, van Berckel BNM, Luurtsema G, Lubberink M, Geldof AA, Bosch TM, et al. Effect of age on functional P-glycoprotein in the blood–brain barrier measured by use of (R)-[11C]verapamil and positron emission tomography. Clin Pharmacol Ther. 2006;79:540–8.
van Assema DME, Lubberink M, Boellaard R, Schuit RC, Windhorst AD, Scheltens P, et al. P-glycoprotein function at the blood–brain barrier: effects of age and gender. Mol Imaging Biol. 2012;14:771–6.
Kervezee L, Hartman R, van den Berg D-J, Shimizu S, Emoto-Yamamoto Y, Meijer JH, et al. Diurnal variation in P-glycoprotein-mediated transport and cerebrospinal fluid turnover in the brain. AAPS J. 2014;16:1029–37.
Savolainen H, Meerlo P, Elsinga PH, Windhorst AD, Dierckx RAJO, Colabufo NA, et al. P-glycoprotein function in the rodent brain displays a daily rhythm, a quantitative in vivo PET study. AAPS J. 2016;18:1524–31.
Vendelbo J, Olesen RH, Lauridsen JK, Rungby J, Kleinman JE, Hyde TM, et al. Increasing BMI is associated with reduced expression of P-glycoprotein (ABCB1 gene) in the human brain with a stronger association in African Americans than Caucasians. Pharmacogenomics J. 2018;18:121–6.
Banks WA. The blood–brain barrier interface in diabetes mellitus: dysfunctions, mechanisms and approaches to treatment. Curr Pharm Des. 2020;26:1438–47.
Strock SE, Hartz AMS, Bernard J, Wolf A, Kachlmeier A, Mahringer A, et al. The concerted amyloid-beta clearance of LRP1 and ABCB1/P-gp across the blood-brain barrier is linked by PICALM. Brain Behav Immun. 2018;73:21–33.
Sanchez-Covarrubias L, Slosky LM, Thompson BJ, Davis TP, Ronaldson PT. Transporters at CNS barrier sites: obstacles or opportunities for drug delivery? Curr Pharm Des. 2014;20:1422–49.
Sita G, Hrelia P, Tarozzi A, Morroni F. P-glycoprotein (ABCB1) and oxidative stress: focus on Alzheimer’s disease. Oxid Med Cell Longev. 2017;2017:7905486.
Alasmari F, Ashby CR Jr, Hall FS, Sari Y, Tiwari AK. Modulation of the ATP-binding cassette B1 transporter by neuro-inflammatory cytokines: role in pathogenesis of Alzheimer’s disease. Front Pharmacol. 2018;9:658.
Pugazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathway in obesity, diabetes, and Alzheimer’s disease. Biochem Biophys Acta Mol Basis Dis. 2017;1863:1037–45.
Goldwaser EL, Acharya NK, Sarkar A, Godsey G, Nagele RG. Breakdown of the cerebrovasculature and blood–brain barrier: a mechanistic link between diabetes mellitus and Alzheimer’s disease. J Alzheimers Dis. 2016;54:445–56.
Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, et al. Functional polymorphisms of the human multidrug resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473–8.
Takano A, Kusuhara H, Suhara T, Ieiri I, Morimoto T, Lee Y-J, et al. Evaluation of in vivo P-glycoprotein function at the blood–brain barrier among MDR1 gene polymorphisms by using 11C-verapamil. J Nucl Med. 2006;47:1427–33.
Brunner M, Langer O, Sunder-Plassmann R, Dobrozemsky G, Müller U, Wadsak W, et al. Influence of functional haplotypes in the drug transporter gene ABCB1 on central nervous system drug distribution in humans. Clin Pharmacol Ther. 2005;78:182–90.
Mossel P, García-Varela L, Arif WM, van der Weijdan CWJ, Boersma HH, Willemsen ATM, et al. Evaluation of P-glycoprotein function at the blood–brain barrier using [18F]MC225 PET. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05419-8.
García-Varela L, Rodríguez-Pérez M, Custoida A, Moraga-Amaro R, Colabufo NA, Aguiar P, et al. In vivo induction of P-glycoprotein function can be measured with [18F]MC225 and PET. Mol Pharm. 2021. https://doi.org/10.1021/acs.molpharmaceut.1c00302.
Acknowledgements
We thank Mr. Masanari Sakai and Mr. Kosuke Nishino for technical support with cyclotron operation and radiosynthesis, Ms. Kimiko Yokoyama and Ms. Kazuko Takagi for care of the subjects during PET scanning and Ms. Airin Onishi for coordination of the clinical study.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research (C) Nos. 18K07658 and 21K07663 from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All procedures performed in studies involving human participants were approved by the Ministry of Health, Labour and Welfare Certified Clinical Research Review Board, Tokyo Metropolitan Geriatric Medical Center (CRB3180026) and in accordance with the principles of the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Written informed consent was obtained from all individual participants prior to their inclusion in the study.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Toyohara, J., Sakata, M., Ishibashi, K. et al. First clinical assessment of [18F]MC225, a novel fluorine-18 labelled PET tracer for measuring functional P-glycoprotein at the blood–brain barrier. Ann Nucl Med 35, 1240–1252 (2021). https://doi.org/10.1007/s12149-021-01666-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01666-9