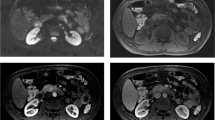
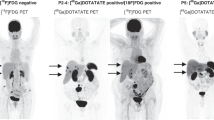
Abstract
Objective
68Ga-DOTATATE positron emission tomography/computed tomography (PET/CT) is a useful tool for diagnosing and staging neuroendocrine neoplasms (NEN). Unlike other PET tracers like FDG, the meaningfulness and use of standardized uptake values (SUVs) of 68Ga-DOTATATE is not well-established. This study aimed to determine if a correlation exists between intensity of 68Ga-DOTATATE uptake and markers of cellular proliferation.
Methods
This retrospective study included 79 patients with positive 68Ga-DOTATATE PET/CT and Ki-67 and/or mitotic index (MI) available on pathology report. SUVmax of the most intense lesion and the most intense organ-matched lesion were determined. Demographics and pathology results for Ki-67 and MI were collected from the electronic medical record. Correlations and trends for correlations of SUVmax to Ki-67 and MI were performed using Kruskal–Wallis and Cuzick trend tests.
Results
A trend for an association between SUVmax and Ki-67 grade was found; median SUVmax of Ki-67 < 3%, 3–20%, and > 20% was 35.2, 31.8, and 12.8 (p = 0.077), respectively. There was also a trend between SUVmax and Ki-67 categories in organ-matched lesions (p = 0.08). The median organ-matched SUVmax of MI < 2, 2–20, and > 20 lesions was 34.2, 18, and 21.7, respectively, (Cuzick trend test p = 0.066). The median SUVmax for small bowel, pancreatic, and other primary locations was 27.6, 46.9, and 9.3 (p < 0.01), respectively.
Conclusions
The association between 68Ga-DOTATATE SUVmax, histologic grade, and primary site of NEN demonstrates its potential use for prognostication, or potentially as a surrogate for histologic grading when biopsy is not possible.






Similar content being viewed by others
References
Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80(Suppl 1):3–7.
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–42.
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–72.
Ozaslan E, Bayram F, Karaca H, Gursoy S, Ozturk F, Sozuer E, et al. Best prognostic factor of neuroendocrine tumors: grade or stage? A multidisciplinary single-center study. Turk J Gastroenterol. 2016;27(6):509–14.
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–8.
Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–86.
Halfdanarson TR, Strosberg JR, Tang L, Bellizzi AM, Bergsland EK, O’Dorisio TM, et al. The North American neuroendocrine tumor society consensus guidelines for surveillance and medical management of pancreatic neuroendocrine tumors. Pancreas. 2020;49(7):863–81.
Strosberg JR, Halfdanarson TR, Bellizzi AM, Chan JA, Dillon JS, Heaney AP, et al. The North American neuroendocrine tumor society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46(6):707–14.
WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. In: Travis WD, Brambilla E, Burke AP, et al., editors. 4 ed. Lyon: IARC; 2015.
Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34(7):982–93.
Khan MU, Khan S, El-Refaie S, Win Z, Rubello D, Al-Nahhas A. Clinical indications for Gallium-68 positron emission tomography imaging. Eur J Surg Oncol. 2009;35(6):561–7.
Reubi JC, Schär JC, Waser B, Wenger S, Heppeler A, Schmitt JS, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27(3):273–82.
Deppen SA, Blume J, Bobbey AJ, Shah C, Graham MM, Lee P, et al. 68Ga-DOTATATE compared with 111In-DTPA-octreotide and conventional imaging for pulmonary and gastroenteropancreatic neuroendocrine tumors: a systematic review and meta-analysis. J Nucl Med. 2016;57(6):872–8.
Deppen SA, Liu E, Blume JD, Clanton J, Shi C, Jones-Jackson LB, et al. Safety and efficacy of 68Ga-DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. J Nucl Med. 2016;57(5):708–14.
Geijer H, Breimer LH. Somatostatin receptor PET/CT in neuroendocrine tumours: update on systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2013;40(11):1770–80.
Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ. High management impact of 68Ga-DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56(1):40–7.
Krenning EP, Valkema R, Kooij PP, Breeman WA, Bakker WH, deHerder WW, et al. Scintigraphy and radionuclide therapy with [indium-111-labelled-diethyl triamine penta-acetic acid-D-Phe1]-octreotide. Ital J Gastroenterol Hepatol. 1999;31(Suppl 2):S219–23.
Cherk MH, Kong G, Hicks RJ, Hofman MS. Changes in biodistribution on (68)Ga-DOTA-Octreotate PET/CT after long acting somatostatin analogue therapy in neuroendocrine tumour patients may result in pseudoprogression. Cancer Imaging. 2018;18(1):3.
Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16(3):978–85.
Binderup T, Knigge U, Loft A, Mortensen J, Pfeifer A, Federspiel B, et al. Functional imaging of neuroendocrine tumors: a head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scintigraphy, and 18F-FDG PET. J Nucl Med. 2010;51(5):704–12.
Bucau M, Laurent-Bellue A, Poté N, Hentic O, Cros J, Mikail N, et al. 18F-FDG uptake in well-differentiated neuroendocrine tumors correlates with both Ki-67 and VHL pathway inactivation. Neuroendocrinology. 2018;106(3):274–82.
Ambrosini V, Campana D, Polverari G, Peterle C, Diodato S, Ricci C, et al. Prognostic value of 68Ga-DOTANOC PET/CT SUVmax in patients with neuroendocrine tumors of the pancreas. J Nucl Med. 2015;56(12):1843–8.
Campana D, Ambrosini V, Pezzilli R, Fanti S, Labate AM, Santini D, et al. Standardized uptake values of (68)Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med. 2010;51(3):353–9.
Kaewput C, Suppiah S, Vinjamuri S. Correlation between standardized uptake value of (68)Ga-DOTA-NOC positron emission tomography/computed tomography and pathological classification of neuroendocrine tumors. World J Nucl Med. 2018;17(1):34–40.
Partelli S, Rinzivillo M, Maurizi A, Panzuto F, Salgarello M, Polenta V, et al. The role of combined Ga-DOTANOC and (18)FDG PET/CT in the management of patients with pancreatic neuroendocrine tumors. Neuroendocrinology. 2014;100(4):293–9.
Boy C, Heusner TA, Poeppel TD, Redmann-Bischofs A, Unger N, Jentzen W, et al. 68Ga-DOTATOC PET/CT and somatostatin receptor (sst1-sst5) expression in normal human tissue: correlation of sst2 mRNA and SUVmax. Eur J Nucl Med Mol Imaging. 2011;38(7):1224–36.
Chan H, Moseley C, Zhang L, Bergsland EK, Pampaloni MH, Van Loon K, et al. Correlation of DOTATOC uptake and pathologic grade in neuroendocrine tumors. Pancreas. 2019;48(7):948–52.
Miederer M, Seidl S, Buck A, Scheidhauer K, Wester HJ, Schwaiger M, et al. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging. 2009;36(1):48–52.
Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1, Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112(11):2447–55.
Yu J, Li N, Li J, Lu M, Leal JP, Tan H, et al. The correlation between [(68)Ga]DOTATATE PET/CT and cell proliferation in patients with GEP-NENs. Mol Imaging Biol. 2019;21(5):984–90.
Panagiotidis E, Alshammari A, Michopoulou S, Skoura E, Naik K, Maragkoudakis E, et al. Comparison of the impact of 68Ga-DOTATATE and 18F-FDG PET/CT on clinical management in patients with neuroendocrine tumors. J Nucl Med. 2017;58(1):91–6.
Strosberg J, Nasir A, Coppola D, Wick M, Kvols L. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40(9):1262–8.
Khan MS, Luong TV, Watkins J, Toumpanakis C, Caplin ME, Meyer T. A comparison of Ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms. Br J Cancer. 2013;108(9):1838–45.
McCall CM, Shi C, Cornish TC, Klimstra DS, Tang LH, Basturk O, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol. 2013;37(11):1671–7.
Klimstra DSKG, La Rosa S, Rindi G. Classification of neuroendocrine neoplasms of the digestive system. In: Board W, editor. WHO classification of tumours: digestive system tumors. 5th ed. Lyon: International Agency for Research on Cancer; 2019. p. 16.
O’Toole D, Saveanu A, Couvelard A, Gunz G, Enjalbert A, Jaquet P, et al. The analysis of quantitative expression of somatostatin and dopamine receptors in gastro-entero-pancreatic tumours opens new therapeutic strategies. Eur J Endocrinol. 2006;155(6):849–57.
Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. Am J Roentgenol. 2010;195(2):310–20.
Focke CM, Bürger H, van Diest PJ, Finsterbusch K, Gläser D, Korsching E, et al. Interlaboratory variability of Ki67 staining in breast cancer. Eur J Cancer. 2017;84:219–27.
Alkhasawneh A, Reith JD, Toro TZ, Ayed AO, Lu X, George TJ, et al. Interobserver variability of mitotic index and utility of PHH3 for risk stratification in gastrointestinal stromal tumors. Am J Clin Pathol. 2015;143(3):385–92.
Qian ZR, Li T, Ter-Minassian M, Yang J, Chan JA, Brais LK, et al. Association between somatostatin receptor expression and clinical outcomes in neuroendocrine tumors. Pancreas. 2016;45(10):1386–93.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Heather Jacene discloses Advanced Accelerator Applications—consulting fees, American Roentgen Ray Society—stipend, Australian and New Zealand Urogenital and Prostate Cancer Clinical Trial Group—honoraria, Cambridge University Press—royalties, Janssen—honoraria, Siemens Healthcare, GTx, Inc, Blue Earth Diagnostics, Inc—research support to institution. Dr. Jennifer Chan discloses—consulting for Advanced Accelerator Applications, Lexicon, Ipsen and Stock Ownership: Merck. The rest of the authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karls, S., Gold, R., Kravets, S. et al. Correlation of 68Ga-DOTATATE uptake on PET/CT with pathologic features of cellular proliferation in neuroendocrine neoplasms. Ann Nucl Med 35, 1066–1077 (2021). https://doi.org/10.1007/s12149-021-01642-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01642-3