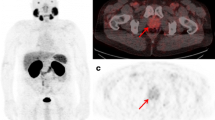
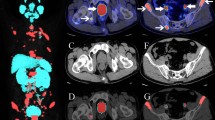
Abstract
Background
68 Ga-PSMA-PET has an increasing importance in the evaluation of prostate cancer patients due to its high sensitivity and specificity in identifying neoplastic lesions in the clinical setting of elevated prostate-specific antigen (PSA). The objective of this study was to calculate the whole-body tumor burden using volumetric quantification of lesions detected in 68Ga-PSMA-PET of prostate cancer patients with biochemical recurrence and correlate these findings with clinical and image parameters.
Methods
Each patient had their 68Ga-PSMA-PET analyzed for the presence of neoplastic lesions. Their PSA levels and clinical information were recorded. In positive cases, the tumor burden (TL-PSMA) was calculated with a semi-automatic software and manually, and the results are analyzed and tested.
Results
We analyzed 100 prostate cancer patients, mean age of 69.9 ± 9.7 years and a median PSA of 1.73 ng/dL. 68Ga-PSMA-PET identified neoplastic lesions in 72% of them. The median TL-PSMA was 55.95 ml (1.1–28,080 ml). TL-PSMA and PSA were strongly correlated (rho = 0.71, p < 0.0001, 95% CI 0.60–0.80). TL-PSMA and PSA levels groups had a significant correlation and TL-PSMA and Gleason score were independent variables associated with PSA levels (p < 0.05).
Conclusion
TL-PSMA strongly and independently correlates with PSA levels in prostate cancer patients and could be used as a biomarker to separate them into groups with high or low tumor burden, instead of considering only the number of lesions.




Similar content being viewed by others
References
Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42.
Tilki D, Kim S, Il HuB, Dall’Era MA, Evans CP. Ultrasensitive prostate specific antigen and its role after radical prostatectomy: a systematic review. J Urol. 2015;193:1525–31.
Einspieler I, Rauscher I, Düwel C, Krönke M, Rischpler C, Habl G, et al. Detection efficacy of hybrid 68 Ga-PSMA Ligand PET/CT in prostate cancer patients with biochemical recurrence after primary radiation therapy defined by phoenix criteria. J Nucl Med. 2017;58:1081–7.
Lin C, Itti E, Haioun C, Petegnief Y, Luciani A, Dupuis J, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med. 2007;48:1626–32.
Carkaci S, Sherman CT, Ozkan E, Adrada BE, Wei W, Rohren EM, et al. 18F-FDG PET/CT predicts survival in patients with inflammatory breast cancer undergoing neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2013;40:1809–16.
Marinelli B, Espinet-Col C, Ulaner GA, McArthur HL, Gonen M, Jochelson M, et al. Prognostic value of FDG PET/CT-based metabolic tumor volumes in metastatic triple negative breast cancer patients. Am J Nucl Med Mol Imaging. 2016;6:120–7.
Kaira K, Higuchi T, Naruse I, Arisaka Y, Tokue A, Altan B, et al. Metabolic activity by 18F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45:56–66.
Etchebehere EC, Araujo JC, Milton DR, Erwin WD, Wendt RE, Swanston NM, et al. Skeletal tumor burden on baseline 18F-fluoride PET/CT predicts bone marrow failure after 223Ra therapy. Clin Nucl Med. 2016;41:268–73.
Rohren EM, Etchebehere EC, Araujo JC, Hobbs BP, Swanston NM, Everding M, et al. Determination of skeletal tumor burden on 18F-fluoride PET/CT. J Nucl Med. 2015;56:1507–12.
Schmidkonz C, Cordes M, Schmidt D, Bäuerle T, Goetz TI, Beck M, et al. 68 Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:1862–72.
Schmuck S, von Klot CA, Henkenberens C, Sohns JM, Christiansen H, Wester H-J, et al. Initial experience with volumetric 68 Ga-PSMA I&T PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer. J Nucl Med. 2017;58:1962–8.
Hirata K, Kobayashi K, Wong K-P, Manabe O, Surmak A, Tamaki N, et al A Semi-Automated Technique Determining the Liver Standardized Uptake Value Reference for Tumor Delineation in FDG PET-CT. Chen K, editor. PLoS One. 2014;9:e105682.
von Eyben FE, Picchio M, von Eyben R, Rhee H, Bauman G. 68 Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur Urol Focus Eur Assoc Urol. 2018;4:686–93.
Etchebehere EC, Araujo JC, Fox PS, Swanston NM, Macapinlac HA, Rohren EM. Prognostic factors in patients treated with 223Ra: the role of skeletal tumor burden on baseline 18F-fluoride PET/CT in predicting overall survival. J Nucl Med. 2015;56:1177–84.
Zacho HD, Nielsen JB, Afshar-Oromieh A, Haberkorn U, DeSouza N, De Paepe K, et al. Prospective comparison of 68 Ga-PSMA PET/CT, 18F-sodium fluoride PET/CT and diffusion weighted-MRI at for the detection of bone metastases in biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:1884–97.
Dyrberg E, Hendel HW, Huynh THV, Klausen TW, Løgager VB, Madsen C, et al. 68 Ga-PSMA-PET/CT in comparison with 18F-fluoride-PET/CT and whole-body MRI for the detection of bone metastases in patients with prostate cancer: a prospective diagnostic accuracy study. Eur Radiol. 2019;29:1221–30.
Kyriakopoulos CE, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080–7.
Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. RadioGraphics. 2018;38:200–17.
Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:678–88.
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Acknowledgements
We thank the Real Nuclear team for their support during this project.
Funding
None. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The local Institutional Review Board approved this study. Since this was a retrospective study with secondary data, the Local Ethics Committee did not request individual informed consent. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brito, A.E.T., Mourato, F.A., de Oliveira, R.P.M. et al. Evaluation of whole-body tumor burden with 68Ga-PSMA PET/CT in the biochemical recurrence of prostate cancer. Ann Nucl Med 33, 344–350 (2019). https://doi.org/10.1007/s12149-019-01342-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-019-01342-z