Abstract
Objective
The objective of this study was to determine if clinical dynamic PET/CT imaging with 11C-L-methyl-methionine (11C-MET) in healthy older women can provide an estimate of tissue-level post-absorptive and post-prandial skeletal muscle protein synthesis that is consistent with the more traditional method of calculating fractional synthesis rate (FSR) of muscle protein synthesis from skeletal muscle biopsies obtained during an infusion of L-[ring 13C6] phenylalanine (13C6-Phe).
Methods
Healthy older women (73 ± 5 years) completed both dynamic PET/CT imaging with 11C-MET and a stable isotope infusion of 13C6-Phe with biopsies to measure the skeletal muscle protein synthetic response to 25 g of a whey protein supplement. Graphical estimation of the Patlak coefficient Ki from analysis of the dynamic PET/CT images was employed as a measure of incorporation of 11 C-MET in the mid-thigh muscle bundle.
Results
Post-prandial values [mean ± standard error of the mean (SEM)] were higher than post-absorptive values for both Ki (0.0095 ± 0.001 vs. 0.00785 ± 0.001 min−1, p < 0.05) and FSR (0.083 ± 0.008 vs. 0.049 ± 0.006%/h, p < 0.001) in response to the whey protein supplement. The percent increase in Ki and FSR in response to the whey protein supplement was significantly correlated (r = 0.79, p = 0.015).
Conclusions
Dynamic PET/CT imaging with 11C-MET provides an estimate of the post-prandial anabolic response that is consistent with a traditional, invasive stable isotope, and muscle biopsy approach. These results support the potential future use of 11C-MET imaging as a non-invasive method for assessing conditions affecting skeletal muscle protein synthesis.
Similar content being viewed by others
Introduction
Muscle-wasting conditions place a significant burden on the public health system and are a threat to independence and quality of life [1, 2]. Our ability to develop pharmacologic, nutritional, or exercise based countermeasures to muscle/function loss is intrinsically tied to the availability of quantitative methods to characterize their effects on the physical and biochemical properties of skeletal muscle tissue.
In research environments, the measurement of acute localized skeletal muscle protein fractional synthesis rate (FSR) is traditionally used to quantify the response to an anabolic stimulus [3, 4]. This moderately invasive procedure includes a stable isotope infusion (e.g., L- [ring-13C6] phenylalanine (13C-Phe)), peripheral venous blood sampling, and serial needle muscle biopsies, typically from the vastus lateralis. The stable isotope, 3-pool modeling technique provides an estimate of protein synthesis and breakdown, but requires femoral venous and arterial catheterization [5]. While these methodologies continue to be used successfully in a variety of clinical research environments [6, 7], they may be contraindicated for clinically vulnerable populations. Thus, a technology to rapidly and non-invasively evaluate skeletal muscle protein anabolism would have significant impact on the assessment of muscle-wasting and associated countermeasures in people unable to tolerate prolonged, more invasive studies.
In the late 1990s, Fischman and colleagues [8] introduced dynamic positron emission tomography (PET) imaging of skeletal muscle [8, 9]. They combined an infusion of 11C-L-Methyl-Methionine (11C-MET) with three compartment kinetic modeling to provide a minimally invasive, in vivo estimate of skeletal muscle protein synthesis (MPS) in potentially any muscle bed, at any anatomic site. An animal study demonstrated that uptake of 11C-MET in skeletal muscle is highly correlated with its stable isotope analog (13C-L-Methyl-Methionine) [9], and basal (i.e., fasted and resting) kinetic model parameters have successfully been evaluated in adult humans [8]. However, until recently, there were no data evaluating the ability of PET imaging to quantify MPS changes in response to known anabolic stimuli. In 2012, Harnish et al. showed that 11C-MET responded to the anabolic stimulus of exercise-induced muscle fatigue [10]. In this study of unilateral knee extension and flexion, 11C-MET uptake was increased 1-h post-exercise in the exercised compared to the control leg. This was reflected in a side-to-side difference in the Patlak Coefficient (K i ), a measure of forward extraction of the imaging agent into the skeletal muscle protein compartment [10].
The studies referenced above support the potential to use non-invasive imaging methodology to assess the anabolic response of skeletal muscle to clinically relevant nutritional and pharmacologic interventions used to treat skeletal muscle atrophy in patient groups such as sarcopenic elderly, and patients with muscle-wasting conditions such as chronic kidney disease, HIV, or diabetes. However, to be considered for evaluation of MPS in these populations, it is important to show that measures derived from imaging correlate with information coming from gold standard techniques such as 13C-Phe infusion/biopsy. Although PET/CT and stable isotope infusion/biopsies use different tracers (11C-MET and 13C-Phe) to measure Ki and FSR, respectively, both methodologies effectively target the biological process of protein synthesis by quantifying the incorporation of an isotopically labeled amino acid into the skeletal muscle tissue. Therefore, the goal of this study was to determine feasibility of clinical dynamic PET/CT imaging with 11C-MET in healthy older women to provide an estimate of tissue-level post-absorptive and post-prandial skeletal MPS that is consistent with FSR calculated from skeletal muscle biopsies obtained during an infusion of 13C-Phe. We chose to use older women for this current study, because they tend to be at high risk for sarcopenia [11], but are anecdotally less likely to volunteer for biopsy studies and are more difficult to biopsy because of lower lean mass, making them a population that would benefit from the development of non-invasive methodology.
Materials and methods
Subjects
Healthy, community-dwelling, post-menopausal, tracer naïve women aged 66–80 years (average age 73 ± 5 years; n = 13) were recruited from the greater San Francisco Bay Area. Study protocols and procedures were conducted in accordance with the Declaration of Helsinki and reviewed and approved by the Institutional Review Board of the University of California San Francisco (UCSF). After providing written informed consent, volunteers were medically screened in the UCSF Clinical Research Center (CRC). Subjects were excluded if they had been diagnosed with any musculoskeletal or metabolic disease, including diabetes, uncontrolled hypertension, or autoimmune disease, as previously described [12].
Procedures
All subjects completed three study visits in sequential order, with each visit separated by 1–2 weeks. The protocols to measure changes in Ki (PET/CT) required two imaging visits, one in which the subjects were imaged after an overnight fast (water only) and one in which the subjects arrived in the fasting state and then consumed a 25-g whey protein isolate supplement (BiPro, Davisco, Le Sueur, MN) 1 h prior to imaging. The FSR (muscle biopsy) protocol involved an infusion of 13C-Phe during which biopsies were obtained immediately before and 2 h after ingestion of the whey protein supplement. The timing of the imaging and infusion biopsy protocols is summarized in Fig. 1.
PET/CT image acquisition
For the imaging visits, subjects reported to the China Basin Outpatient Imaging Center at the UCSF Department of Radiology and Biomedical Imaging. All images were acquired using a Discovery VCT PET/CT camera (GE Healthcare, Chicago, IL, USA). Subject was oriented in the scanner in the supine position, with legs extended into the scanner aperture and feet straight up and taped together. An anteroposterior scout view depicted the lower body anatomy from the iliac crest to the tibial plateau and was employed to determine a CT scanning interval from the mid-thigh to a point roughly 2-cm superior to the distal femoral condyle. The dynamic PET axial field of view, which extended 14.5 cm along the table axis (the fixed width of the PET detector array), was centered in the CT volume. Based on the CT scan volume defined from the scout view, the subject was imaged with a helical CT scan (120 kVp, 300 mAs, 3.75-mm sections, 5122 matrix, and standard reconstruction kernel). After the CT image was acquired, the subject was injected with a bolus of 462-MBq (range 407–481 MBq) 11C-MET solution, resulting in an approximately 2-mSv effective dose to the subjects. Beginning concurrently with the 11C-MET bolus injection, the dynamic PET data were acquired using a sequence of 27 temporally contiguous frames over the course of 60 min. The time frames were of variable durations, acquired in the following sequence: [15 × 10 s, 5 × 30 s, 4 × 5 min, 2 × 10 min, 1 × 15 min]. Images were reconstructed into voxels of dimensions 1.95 × 1.95 × 3.27 mm3 in 256 × 256 × 47 matrices via a 3D ordered subset expectation maximization algorithm (28 subsets, two iterations) with CT-based attenuation correction provided by the scanner manufacturer.
PET/CT image analysis
To determine the rate of incorporation of 11C-MET into bound skeletal muscle protein, we employed the Patlak graphical analysis [13], which can be used to determine the fractional extraction rate K i from plasma into a peripheral compartment (bound protein). The method assumes that the tissue compartment is in rapid equilibrium with the plasma and bound peripheral compartments, and that release from the bound compartment is not observed in the timescale of the study. To derive K i from the dynamic images, the ratio C T(t)/C A(t) is plotted against ∫C A(t)dt/C A(t), integrated between t = 0 and t = 60 min, where C A(t) is the concentration as a function of time t of the tracer 11C-MET in the femoral artery as represented by the image-derived input function (IDIF, see below), and C T(t) is the concentration as a function of time measured in the tissue activity curve (TAC). When the system achieves equilibrium between the three compartments, the Patlak plot becomes linear and the Patlak slope Ki can be derived from fitting that linear component. In a previous study [10], we reported a high correlation (r = 0.92) between K i and the MPS rate estimated by Fishman et al. [8] from kinetic analysis using a three compartment model of dynamic PET data. Because of the robustness and simplicity of the Patlak K i , we utilized it as a measure of image-derived MPS in this study.
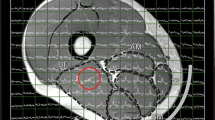
To estimate the contribution of arterial blood flow to the skeletal muscle uptake of 11C-MET as a function of time C A(t), we computed an image-derived arterial input function (IDIF) from region of interest analysis around the femoral artery as previously described [14]. The implementation of the IDIF for analysis of the dynamic images in our study was described by Harnish et al. [10]. To quantify the uptake of 11C-MET in skeletal muscle, 11C-MET concentrations were measured from regions of interest (ROIs) determined automatically from the CT scans using threshold-driven region growing. The distal femoral condyle and the lesser trochanter, two bony landmarks visible on the anteroposterior scout view, were used to establish the inferior and superior limits of the set of axial slices included in the TAC ROI. The inferior limit of the ROI occurred in the axial slice closest to 36% of the distance from the femoral condyle to the lesser trochanter, and the superior limit occurred in the slice closest to 44% of that distance. To avoid contamination of the tissue region by arterial counts, a region surrounding the artery, roughly the extent of the outer radius of the annular region used to subtract tissue background counts from the arterial signal, was excluded from the skeletal muscle tissue ROI. Representative PET/CT images and the region used for derivation of the arterial input function are shown in Fig. 2.
Images from Subject 004. CT (a) and PET (b) images corresponding to central mid-thigh cross section of thigh volume. The fused PET/CT image is shown in (c). The tissue activity curve (TAC) was computed from the region encompassing the total thigh muscle volume, but excluding bone and the region used to determine the image-derived input function (IDIF). These regions are superimposed in gold pixels on all three images
Stable isotope and biopsy methodology
1–2 weeks after the completion of the first imaging visit, volunteers were admitted to the UCSF-CRC. An 18-gauge polyethylene catheter (Insyte-W; Becton Dickinson, Sandy, UT) was inserted into the forearm vein of each arm, one for blood sampling, and one for stable isotope infusion. After baseline blood sampling to measure background phenylalanine enrichment, a primed (2 μmol/kg), constant infusion (0.06 μmol/kg/min) of L- [ring-13C6] phenylalanine was started and continued throughout the study (Fig. 1). A 5-mm Bergstrom needle was used to take a muscle biopsy from the vastus lateralis under local anesthesia following a standardized technique [4]. Subjects ingested 25 g of whey protein mixed with 250 mL of water immediately following the second biopsy. This high-quality protein has been shown to provide a rapid, near maximal increase in skeletal muscle protein synthesis [15]. Peripheral venous blood samples and the final muscle biopsy were obtained according to the schedule outlined in Fig. 1.
Processing of blood and skeletal muscle samples
Venous blood samples were immediately mixed with 1 mL ice-cold sulfosalicylic acid and centrifuged for 20 min (3000 rpm at 4 °C). The supernatant was removed and stored at -80 °C. Muscle samples were blotted and rinsed with saline, flash frozen in liquid nitrogen, and stored at −80 °C. All muscle and blood samples were sent to the University of Texas Medical Branch for analysis. Phenylalanine enrichment of muscle proteins as well as plasma were determined as previously described [7, 12].
Mixed muscle fractional synthesis rate was calculated by measuring the direct incorporation of 13C6-Phe into protein using the precursor-product model [7]:
where E B is the protein-bound phenylalanine enrichment across two sequential biopsies taken at time T1 and T2, respectively, and E P is the adjusted plasma enrichment obtained by dividing venous 13C-Phe enrichment area under the curve between sequential biopsies with the average venous enrichment at each biopsy, as previously described [15, 16]. This adjustment reduces the potential for erroneous underestimation of the post-prandial FSR associated with the depression of plasma phenylalanine enrichment.
Statistical analysis
The anabolic response measured with both PET/CT and stable isotope infusion was analyzed using a paired two-tail student t test with a significant difference set at p < 0.05. Data are presented with the average ± standard error of the mean (SEM). The relationship between baseline Ki and FSR, and between the percent increases in Ki and FSR, measured by PET/CT and stable isotope, respectively, was evaluated using a Pearson correlation. Statistical analyses were performed using the JMP software (Cary, NC).
Based on preliminary work from our group, we expected to be able to detect a significant, 30% increase in Ki with 80% power with a sample size of 12 subjects. Thirteen subjects were recruited for the study, and one subject dropped out of the study after the first imaging visit and was not included in any analysis. Out of the 12 subjects that completed all 3 study visits, 1 subject had invalid post-prandial K i measurement, because the tissue activity curve did not saturate during the course of the imaging study and was not included in the analysis of K i . Furthermore, two subjects had unusable biopsy samples due to a high amount of fat and were excluded from the FSR analysis. Therefore, nine subjects had usable post-absorptive and post-prandial K i and FSR data and were included in the correlation analysis.
Results
Baseline characteristics
Figure 3 shows post-absorptive and post-prandial arterial input and tissue activity curves and Fig. 4 shows Patlak plots for one of the study subjects (Subject 4). Table 1 provides demographic and post-absorptive and post-prandial Ki and FSR values for the subjects. There was no correlation between post-absorptive K i and FSR (r = 0.12, p = 0.75).
Anabolic response
A 25 g whey protein supplement elicited a 20% increase and a 70% increase in K i (Fig. 5a) and FSR (Fig. 5b), respectively. The post-absorptive and post-prandial as well the percent increase are consistent with the previous values reported in the literature [17]. The percent increase in Ki and FSR in response to the whey protein supplement was significantly correlated (Fig. 6).
Box and whiskers plot post-absorptive and post-prandial K i (n = 11; a). Box and whiskers plots of post-absorptive and post-prandial FSR (n = 10; b). For both plots, the box represents the interquartile range containing 50% of the values. The whiskers denote the maximum and minimum values (excluding outliers). The link drawn across the box is the median. Both K i and FSR significantly increased in response to a 25 g whey protein supplement
Discussion
The prevalence of sarcopenia and other clinical conditions are associated with muscle-wasting motivate the development of strategies to reduce or prevent disability. The ultimate goal of such therapies is the preservation/restoration of muscle mass and functional capacity.
Therefore, the ability to quantify acute changes in skeletal muscle protein synthesis is an essential prerequisite to understanding the potential clinical/practical relevance of anabolic therapies or interventions. In this study, we demonstrated that 11C-MET PET/CT imaging shows an anabolic response to a clinically relevant therapeutic approach, dietary protein supplementation, and that the response is correlated to 13C-Phe infusion biopsy. Thus, we provided new evidence that a non-invasive quantitative imaging approach can provide valuable new information to assess the potential effects of approaches to counter muscle atrophy.
The historic gold standard for assessing skeletal muscle protein synthesis involves the infusion of a stable isotope labeled amino acid (e.g., 13C6- phenylalanine, L-[ring-2H5]phenylalanine, and L-[1-13C]leucine) coupled with serial muscle biopsies along with peripheral vein and occasionally femoral artery and vein blood sampling [5]. While this technique is sensitive and widely used, it has several limitations: (1) the quantity of tissue obtained by biopsy is limited (50–100 mg /muscle biopsy); (2) not all muscle beds/sites can be safely or effectively biopsied; (3) the procedure is moderately invasive, involves some discomfort, and results in a small scar; (4) obtaining suitable quality and quantity of muscle tissue from sarcopenic, frail, and/or obese individuals is challenging; (5) quantifying a change in fractional synthesis rate (FSR) requires at least two biopsies obtained over a 2–4-h period.
There are other methodologies that provide some insight into skeletal muscle response to anabolic therapies. Change in serum albumin is used as a crude clinical marker of muscle mass status [18]. Whole-body rate of appearance (Ra)/rate of disappearance (Rd) studies using stable isotopes are very useful in clinical populations, because they do not require biopsies [19]. However, the gut/splanchnic bed plays a dominant role in determining whole-body Ra/Rd and, therefore, limits the ability to determine an isolated skeletal muscle response. Deuterium-labeled water is a minimally invasive method of assessing whole-body protein turnover [20], but is not as specific as FSR, especially when measuring an acute anabolic response. Nitrogen balance studies are traditionally used for determining protein needs [21], but have limited clinical utility because of the complexity and time-intensive nature of the method.
Imaging approaches such as 11C-MET PET/CT imaging offer some time savings and clinical advantages. Unlike a traditional stable isotope-based infusion/biopsy process, in which two or more hours of the initial infusion are required to establish an isotopic steady state, the imaging approach requires roughly 1 h to obtain a set of image frames that can be analyzed to obtain the Patlak coefficient, which describes the forward transport and capture of the 11C from the blood pool into the muscle tissue bed. While other techniques such as the stable isotope pulse bolus technique are shorter than the traditional infusion protocol, it is not designed to measure the response to an anabolic stimulus [22]. In addition to time savings, PET/CT imaging is less invasive, and involves considerably less discomfort than serial skeletal muscle biopsies. Furthermore, the 11C-MET PET/CT approach can be carried out by a nuclear medicine technologist and does not require skilled nursing and physician support to perform the injection and imaging. Finally, the 11C-MET PET/CT image, as acquired in our study, provided a 3D map of 11C-MET uptake in a 14-cm thick volume of the thigh imaged as a stack of slices with 3.75-mm thickness. Compared to the biopsy of the vastus lateralis, this allowed us to average the Ki value over a large tissue volume, reducing susceptibility to tissue sampling variations. Because of this capability, PET/CT provides functional information about the general distribution of an anabolic response across an entire limb containing an array of different functional muscle groups that must operate synergistically. Our findings suggest that PET/CT imaging may function synergistically with biopsy, by focusing the use of the biopsy sample for the purposes for which it is uniquely suited, for example for studies of myofibrillar structure or of gene expression.
However, it is important to note that this approach has technical limitations as well as strengths. One limitation is that this type of study necessarily involves radiation exposure due to the administration of 11C-MET. In our case, the total radiation exposure associated with the two PET/CT studies was approximately 4 mSv, which is equivalent to slightly more than 1 year of background exposure. Another limitation of the approach is that in contrast to biopsy methods, it is not possible, due to the limited imaging resolution, to quantify 11C-MET uptake in particular compartments of the skeletal muscle tissue, such as mitochondrial or myofibrillar protein. Thus, although it offers advantages in the rapidity of acquisition and reduced invasiveness, as well as ability to survey whole muscle beds, increases in 11C-MET uptake represent a relatively crude measure of anabolic response of skeletal muscle tissue. In addition to technical limitations, there are also logistical and economic characteristics that should be considered. Although PET/CT systems are widely distributed throughout the United States (≅1300 systems [23]), 11C-MET imaging requires access to an onsite cyclotron and radiochemistry facility, and thus tends to be limited to a smaller number academic medical centers. Thus, based on this combination of advantages and limitations, we believe that at present, the primary utility of this approach is as a research tool, concentrated in academic medical centers where investigations of skeletal muscle function response are most likely to occur.
The current study is the first to show PET/CT imaging of radio-labeled amino acids such as 11-C-MET has an acute skeletal muscle anabolic response to administration of dietary protein and that the extent of the response is correlated to that of the 13C-Phe infusion/biopsy measurements, the gold standard. The previous literature has shown that 11C-MET uptake as measured by PET tracks with the accumulation of the 13 C labeled analog in animal necropsy models [9] and that reasonable values of PSR could be estimated in humans from PET images [8]. In a subsequent study using PET/CT, we showed that K i , which is highly correlated to PSR, increased in relation to exercise in a unilateral leg resistance exercise model [10]. However, that study had limited clinical relevance, because anabolic therapies in sarcopenic subjects are likely to be pharmacologic or nutritional and because that study did not show correlation to a gold standard PSR measurement, stable isotope infusion of 13 C-phenyalanine with serial biopsies. The strength of this study was that it used a nutritional intervention known to result in increased protein synthesis rate, and compared changes in image-derived kinetics of 11C-MET to changes in 13C-Phe kinetics using standard infusion/biopsy approach. We revealed that both techniques showed a significant elevation with the known anabolic stimulus and that the responses of the two approaches were correlated with statistical significance. Interestingly, despite the correlation of percentage change measures, the baseline values of K i and FSR were uncorrelated. We believe that this may arise from differences in the functional roles of methionine and phenyalanine. While both are structural constituents of skeletal muscle protein, methionine is the only sulfur-containing amino acid and has important metabolic and signaling roles [24, 25] apart from its structural role. Unlike phenyalanine, methionine is not produced in the body and must be obtained from external sources. Thus, because there may be inter-individual variations in MET metabolism due to these additional metabolic and signaling roles, skeletal muscle uptake of these two amino acids may be uncorrelated in the fasted state, but be correlated in their responses to a powerful anabolic stimulus that increases incorporation of all amino-acid constituents into skeletal muscle. In summary, from our current findings, and from the previous study showing 11C-MET response to exercise, we believe that that this imaging method shows a similar anabolic response of skeletal muscle tissue to anabolic interventions such as nutritional therapies, exercise, and pharmacologic interventions similarly to 13C-Phe infusion and serial biopsy.
While our study has strengths, and the results are strongly supportive of further exploration of this approach in larger studies, there are limitations to consider. The inter-individual variability in K i and in the change in K i was higher than that observed for FSR. Furthermore, the correlation obtained (r = 0.8) was somewhat low. This is not surprising, given that the number of subjects was small, the two imaging measurements were done on different days, the imaging and biopsy studies were also done separately, and they used different compounds for the MPS measurements. Thus, our study does not establish that 11C-MET imaging is a surrogate measure for 13C-Phe infusion/biopsy study in individuals. However, we do believe that this approach demonstrates the feasibility of using the Ki determination from 11C-MET imaging as a measure of skeletal muscle anabolic response in groups of subjects, and greatly supports the use of this technique as a research tool, particularly, as described below, in subjects who may not be able to sustain a prolonged infusion/biopsy study.
We believe that this approach will have advantages as a research tool, making dynamic assessment of skeletal muscle anabolic response more accessible in the target populations expected for anabolic agents. We believe that this approach is best applied to investigations of health conditions that have a deleterious effect on PSR, such as long-term bed rest. For example, bed rest studies of healthy subjects show up to 30% decreases in PSR as measured by 13C-Phe infusion and biopsy. People, especially the elderly, who may under undergo 2–3 weeks of bed rest for health conditions often suffer from sarcopenia [26–28], which complicates rehabilitation. Because this approach greatly reduces the time burden and discomfort for the patient, 11C-MET imaging with PET/CT may be useful to characterize skeletal muscle deficits in this population. By the same token, it will also be applicable to investigate skeletal muscle function in other disease groups, such as chronic kidney disease [29–31], HIV [32, 33] or diabetic patients [34–36]. Moreover, because the PET image is co-registered with a CT image, it is possible to correlate the Ki measure with measures of muscle cross-sectional area and lean tissue Hounsfield Unit, a measure of fatty infiltration which is associated with altered muscle strength and physical function.
In conclusion, PET/CT with 11C-MET can be used to represent the aggregate response of a group of subjects to conditions that stimulate or reduce protein synthesis. From the previous work, the Ki responds to unilateral leg extension and flexion exercise, which stimulates protein synthesis rate as measured in biopsy/infusion studies. From this work, there is a significant positive response to whey protein ingestion, which agrees with the aggregate response in our subjects by biopsy.
Abbreviations
- FSR:
-
Fractional synthetic rate
- Ki :
-
Patlak Coefficient, representing fractional extraction rate Ki from plasma into a peripheral compartment (bound protein)
- 13C-Phe:
-
L- [ring-13C6] phenylalanine
- PET:
-
Positron emission tomography
- 11C-MET:
-
11C-L-methyl-methionine
- MPS:
-
Muscle protein synthesis
- PSR:
-
Protein synthetic rate
- CT:
-
Computed tomography
- IDIF:
-
Image-derived input function
- TAC:
-
Tissue activity curve
- CA :
-
Activity of 11 C-MET measured in femoral artery by IDIF analysis
- CT :
-
Activity of 11 C-MET measured in skeletal muscle tissue at each point in the TAC
References
Berger MJ, Doherty TJ. Sarcopenia: prevalence, mechanisms, and functional consequences. Interdiscip Top Gerontol. 2010;37:94–114.
Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, et al. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9(4):369–83.
Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286(3):E321–E8.
Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35(7):609–16.
Wolfe R. Protein synthesis and breakdown. Radioactive and stable isotope tracers in biodmedicine: principles and practice of kinetic analysis. New York: Wiley-Liss; 1992.
Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85(12):4481–90.
Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, et al. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89(9):4351–8.
Fischman AJ, Yu YM, Livni E, Babich JW, Young VR, Alpert NM, et al. Muscle protein synthesis by positron-emission tomography with L-[methyl-11C]methionine in adult humans. Proc Natl Acad Sci USA. 1998;95(22):12793–8.
Hsu H, Yu YM, Babich JW, Burke JF, Livni E, Tompkins RG, et al. Measurement of muscle protein synthesis by positron emission tomography with L-[methyl-11C]methionine. Proc Natl Acad Sci USA. 1996;93(5):1841–6.
Harnish R, Streeper T, Saeed I, Schreck C, Dannoon S, Slater J, et al. Quantification of changes in skeletal muscle amino acid kinetics in adult humans in response to exercise via positron-emission tomography with L-[methyl-11C] methionine. J Mol Imag Dynamic. 2012;2(1):103.
Kirchengast S, Huber J. Gender and age differences in lean soft tissue mass and sarcopenia among healthy elderly. Anthropol Anz. 2009;67(2):139–51.
English KL, Mettler JA, Ellison JB, Mamerow MM, Arentson-Lantz E, Pattarini JM, et al. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr. 2016;103(2):465–73.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3(1):1–7.
Croteau E, Lavallee E, Labbe SM, Hubert L, Pifferi F, Rousseau JA, et al. Image-derived input function in dynamic human PET/CT: methodology and validation with 11 C-acetate and 18F-fluorothioheptadecanoic acid in muscle and 18F-fluorodeoxyglucose in brain. Eur J Nucl Med Mol Imaging. 2010;37(8):1539–50.
Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93(5):997–1005.
Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr. 2009;90(1):106–15.
Markofski MM, Dickinson JM, Drummond MJ, Fry CS, Fujita S, Gundermann DM, et al. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp Gerontol. 2015;65:1–7.
Visser M, Kritchevsky SB, Newman AB, Goodpaster BH, Tylavsky FA, Nevitt MC, et al. Lower serum albumin concentration and change in muscle mass: the health, aging and body composition study. Am J Clin Nutr. 2005;82(3):531–7.
Wagenmakers AJ. Tracers to investigate protein and amino acid metabolism in human subjects. Proc Nutr Soc. 1999;58(4):987–1000.
Gasier HG, Fluckey JD, Previs SF. The application of 2H2O to measure skeletal muscle protein synthesis. Nutr Metab (Lond). 2010;7:31.
Calloway DH, Margen S. Variation in endogenous nitrogen excretion and dietary nitrogen utilization as determinants of human protein requirement. J Nutr. 1971;101(2):205–16.
Zhang XJ, Chinkes DL, Wolfe RR. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am J Physiol Endocrinol Metab. 2002;283(4):E753–E64.
IMV. IMV 2015 PET Imaging Market Summary Report 2015 [Available from: http://www.imvinfo.com/user/documents/content_documents/def_dis/2015_10_12_12_23_01_11_IMV_PET_2015_report_datasheet_Oct_2015.pdf.
Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1(5):228–37.
Stipanuk MH, Caudill MA. Biochemical, physiological, and molecular aspects of human nutrition: Elsevier Health Sciences; 2013.
Bell KE, von Allmen MT, Devries MC, Phillips SM. Muscle disuse as a pivotal problem in sarcopenia-related muscle loss and dysfunction. J Frailty Aging. 2016;5(1):33–41.
Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, et al. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol. 2015;593(18):4259–73.
Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev. 2013;12(4):898–906.
Carrero JJ, Johansen KL, Lindholm B, Stenvinkel P, Cuppari L, Avesani CM. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016;90(1):53–66.
Musso CG, Jauregui JR, Macias Nunez JF. Frailty phenotype and chronic kidney disease: a review of the literature. Int Urol Nephrol. 2015;47(11):1801–7.
Yoshida T, Delafontaine P. Mechanisms of cachexia in chronic disease states. Am J Med Sci. 2015;350(4):250–6.
Mialich MS, Dos Santos AP, da Silva BR, de Paula FJ, Jordao AA, Navarro AM. Relationship between adiposity indices, lipodystrophy, and sarcopenia in HIV-positive individuals with and without lipodystrophy. J Clin Densitom. 2016.
Pinto Neto LF, Sales MC, Scaramussa ES, da Paz CJ, Morelato RL. Human immunodeficiency virus infection and its association with sarcopenia. Braz J Infect Dis Off Publ Braz Soc Infect Dis. 2016;20(1):99–102.
Bianchi L, Volpato S. Muscle dysfunction in type 2 diabetes: a major threat to patient’s mobility and independence. Acta diabetologica. 2016.
Guerrero N, Bunout D, Hirsch S, Barrera G, Leiva L, Henriquez S, et al. Premature loss of muscle mass and function in type 2 diabetes. Diabetes Res Clin Pract. 2016;117:32–8.
Yang R, Zhang Y, Shen X, Yan S. Sarcopenia associated with renal function in the patients with type 2 diabetes. Diabetes Res Clin Pract. 2016;118:121–9.
Acknowledgments
The authors’ responsibilities were as follows—DP-J and TL: designed the research; EA-L, IS, LF, UM, DP-J, RH, RH, YS, HVB, CM-A, MHP, JS and TL: conducted the research; JS and HVB developed and carried out the radiosynthesis protocol; EA-L, DP-J, RH and TL: analyzed the data; EA-L, DP-J and TL: wrote the manuscript; TL: had primary responsibility for final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest. The authors thank Michelle Swenson, Mark Howe, Michael Moles for technical support in PET/CT imaging, Marcos Contreras for preparation of 13C-Phe infusions, and Deanna Sheeley and Wendy Staub for nursing support during the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases R21 AR062479-01A1, the UCSF Clinical and Translational Science Institute UL1TR000004, and the Claude D Pepper Older Americans Independence Center (NIH/National Institute on Aging Grant P30 AG024832).
Additional information
E. J. Arentson-Lantz and I. H. Saeed are co-first authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Arentson-Lantz, E.J., Saeed, I.H., Frassetto, L.A. et al. 11C-L-methyl methionine dynamic PET/CT of skeletal muscle: response to protein supplementation compared to L-[ring 13C6] phenylalanine infusion with serial muscle biopsy. Ann Nucl Med 31, 295–303 (2017). https://doi.org/10.1007/s12149-017-1157-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-017-1157-4