Abstract
Objectives
Although positron emission tomography (PET) using [18F]-fluoro-2-deoxy-d-glucose (18F-FDG) is established as one of the first-choice imaging modalities in the diagnosis of chest malignancies, there are several problems to solve in clinical practice, such as false positive uptake in inflammatory diseases. The aim of this study was to evaluate the clinical usefulness of an amino acid tracer, α-[N-methyl-11C]-methylaminoisobutyric acid (11C-MeAIB), in the diagnosis of chest malignancies, in combination with 18F-FDG.
Setting
Fifty-nine cases (57 patients, 66 ± 12 years old) who consulted to our institution for the wish to receive differential diagnosis of chest diseases were included. Purpose of the studies were as follows: differential diagnosis of newly developed lung nodules, n = 22; newly developed mediastinal lesions, n = 20; and both, n = 17 (including lung cancer: n = 19, lymphoma: n = 1, other cancers: n = 2, sarcoidosis: n = 15, non-specific inflammation: n = 18, other inflammatory: n = 4, respectively). Whole-body static PET or PET/CT scan was performed 20 and 50 min after the IV injection of 11C-MeAIB and 18F-FDG, respectively.
Results
11C-MeAIB uptake of malignant and benign lesions was statistically different both in pulmonary nodules (p < 0.005) and in mediastinal lesions (p < 0.0005). In visual differential diagnosis, 11C-MeAIB showed higher results (specificity: 73 %, accuracy: 81 %), compared to those in 18F-FDG (60, 73 %, respectively). In cases of sarcoidosis, 11C-MeAIB showed higher specificity (80 %) with lower uptake (1.8 ± 0.7) in contrast to the lower specificity (60 %) with higher uptake of 18F-FDG (7.3 ± 4.5).
Conclusions
11C-MeAIB PET/CT was useful in the differential diagnosis of pulmonary and mediastinal mass lesions found on CT. 11C-MeAIB PET or PET/CT showed higher specificity than that of 18F-FDG PET/CT in differentiating between benign and malignant disease. Our data suggest that the combination of 18F-FDG and 11C-MeAIB may improve the evaluation of chest lesions, when CT and 18F-FDG PET/CT are equivocal.
Similar content being viewed by others
Introduction
The positron emission tomography (PET) radiopharmaceutical 2-deoxy-2-[18F]fluoro-d-glucose (18F-FDG) is currently used for diagnostic imaging of a variety of tumors [1]. However, 18F-FDG is known to accumulate in normal structures and in sites of inflammation, reducing its specificity [2–5]. Several investigators have reported that 18F-FDG PET performed to assess for malignancy showed 20–50 % false positive results, with the higher false positive rates found in geographical areas of endemic granulomatous diseases, such as histoplasmosis or tuberculosis [5–8].
A variety of new tracers has been introduced in order to overcome these limitations [9–11]. Amino acid tracers are one promising category. 11C-methionine (11C-MET) has been extensively investigated, though with several drawbacks being revealed [12]. 11C-MET is not stable in vivo, becoming trans-methylated, and losing its 11C moiety as it is metabolized [13]. Several studies have shown that 11C-MET is not tumor-specific, and accumulates in some inflammatory diseases, such as sarcoidosis [14, 15].
[N-methyl-11C]α-methylaminoisobutyric acid (11C-MeAIB) is an artificial amino acid PET tracer, which, unlike 11C-MET, is metabolically stable in vivo [16, 17]. It has been shown that 11C-MeAIB is a useful tracer for measurement of amino acid uptake by skeletal muscle, and in the diagnosis of malignant lymphoma and head and neck cancers [16, 18, 19]. The utility of 11C-MeAIB PET in the diagnosis of chest malignancies has not yet been evaluated.
The purpose of this study is to investigate the efficacy of 11C-MeAIB PET or PET/CT as a diagnostic tool for distinguishing between malignancies and inflammatory diseases, when CT or 18F-FDG PET or PET/CT shows equivocal findings.
Materials and methods
Patients
From March 2009 to September 2012, 59 cases (57 patients; male: 35, female: 22; ranging from 28 to 88 years; mean age: 65.5 ± 12.3) (Table 1, left) of new chest lesions detected by CT scan (21 patients had lung lesions only, 20 patients had mediastinal lesions only, and 18 patients had both lesions) were selected. Two patients received this study protocol twice with 1 year interval or more. Each patient gave written informed consent. The tracer study was approved by our institutional review boards, the Human Study Committee (approval number: #36-04, Mar. 25, 2009) and the Committee for the Clinical Use of Short-Half Life Radioactive Materials (approval number: #2008-01, Nov. 28, 2008).
Inclusion criteria were: (1) a new chest lesion detected on CT scan, suspicious for malignancy, (2) CT (and 18F-FDG PET or PET/CT) showing equivocal findings, with further evaluation requested by referring pulmonologists, and (3) disease confirmed by pathology, or clinical follow-up for more than 12 months after PET studies. Exclusion criteria were: (1) malignant or inflammatory lesions which received treatment within 6 months before 18F-FDG PET or PET/CT, (2) apparent direct invasion of neighboring organs, (3) apparent extra-pulmonary metastatic lesions, and (4) patients who refused to undergo 11C-MeAIB PET or PET/CT. Baseline diseases or detailed background of clinical conditions are shown in Table 1 (left).
The CT scans had been performed as routine clinical studies with a multi-detector row CT scanner, Aquilion 16 (Toshiba, Tokyo, Japan). All patients subsequently underwent both 18F-FDG and 11C-MeAIB PET or PET/CT within 2 weeks of the CT.
PET studies
18F was produced by 18O(p, n) 18F reaction. 18F-FDG was synthesized by the nucleophilic substitution method using an 18F-FDG-synthesizing instrument F-100 (SHI, Tokyo, Japan) and a cyclotron, CYPRIS-325R (SHI, Tokyo, Japan). Otherwise, 18F-FDG was purchased commercially from Nihon-Medi-Physics (Tokyo, Japan).
Production of 11C-MeAIB was based on the method proposed by Någren et al. [20]. The patients fasted for more than 5 h before the injection of 18F-FDG or 11C-MeAIB. Blood glucose levels were measured before the injection of 18F-FDG, and all the patients showed levels of ≤150 mg/dL (95.2 ± 13.1 mg/dL). All subjects underwent two separate scans, one following an intravenous injection of 18FDG (298 ± 68 MBq), and another after the intravenous injection of 11C-MeAIB (512 ± 50 MBq). Whole-body PET image acquisition commenced 50 min after 18F-FDG and 20 min after 11C-MeAIB injection. PET scans were performed either by a whole-body PET scanner, GE Advance (GE Healthcare, Waukesha WI, USA), or by a whole-body PET/CT scanner, Siemens True Point Biograph 16 (Siemens/CTI, Erlangen, Germany).
Image analysis
Visual analysis of each lesion on the two PET scans was performed by two experienced nuclear medicine physicians (RN, TH) provided with clinical information including CT scans and tumor markers. All lesions were graded as positive or negative by consensus of two readers. If a nodule showed similar or lower uptake than that in the upper to middle normal mediastinal tissues, uptake was defined as negative. If a nodule showed higher uptake than that of the normal mediastinal tissues, its uptake was defined as positive. However, high 18F-FDG uptake was sometimes defined as negative in cases of sarcoidosis and other benign entities with a characteristic pattern by consensus of two readers.
Semi-quantitative analysis of 18F-FDG and 11C-MeAIB uptake was also performed. Regions of interest (ROIs) were defined on the target lesions in the transaxial tomograms of PET-only images. PET-to-CT co-registration was performed using automatic rigid/non-rigid body-deformable fusion software: Quantiva/BodyGuide (Tomographix IP Ltd., Toronto, Canada). In PET/CT scans, ROIs were defined and confirmed on the fused PET and CT images (hereafter all scans will be referred to as PET/CT scans, since PET images were always fused to CT images). The standardized uptake value (SUV) was calculated as follows:
where C represents tissue activity concentration measured by PET and ID represents the injected dose. The mean SUV of the normal tissue (lung field and mediastinum) was defined as the SUVmean. The highest SUV of the lesion was defined as the SUVmax.
Statistics
All values are expressed as mean ± SD. All the statistical analyses were performed using statistical software, JMP 8J version (SAS Institute, Cary NC, USA), in which p values <0.05 were considered statistically significant. A comparison between each group was analyzed by the Wilcoxon score for unpaired data. Receiver operating characteristic curve (ROC) analyses for the diagnostic accuracy in 11C-MeAIB PET/CT and 18F-FDG PET/CT were generated using GraphPad Prism ver. 5.0 (GraphPad software, San Diego CA, USA). A comparison of SUVmax between 11C-MeAIB and 18F-FDG in each lesion was analyzed by the Logistic regression.
Results
Table 1 summarizes the patient characteristics. The final diagnosis was confirmed pathologically by surgical resection in 17 cases, while biopsy at bronchoscopy, thoracoscopy, and CT-guided biopsy confirmed diagnosis in 10 cases. Resection or biopsy of lymph nodes at neck or supra-clavicular area was performed in seven cases. Clinical diagnosis was determined by follow-up for at least 12 months in 24 cases. Of the 59 cases in the present study, there were 22 malignant and 20 benign pulmonary nodules in the lungs and 10 malignant and 28 benign mediastinal lesions.
Figure 1 shows a typical malignant case, while Fig. 2 shows a typical benign case; in this instance, sarcoidosis. In Fig. 3, a 18F-FDG-strongly positive lung nodule was diagnosed as sarcoidosis. Equivocal findings of metastatic colon cancer are shown in Fig. 4.
A case of lung cancer. Fifty-nine-year-old asymptomatic male patient. Screening chest X-ray showed abnormal density in the left upper lung zone. The patient underwent a contrast-enhanced chest CT scan, which revealed a nodule in the left apex (a) with probably lymph node (ln) metastasis in the left hilum (b). Bronchoscopic biopsy revealed squamous cell carcinoma (SCC). Both 18F-FDG PET and 11C-MeAIB PET (c, d: MIP image) showed high accumulation in the left upper lobe lesion (SUVmax = 13.2 for 18FDG, 6.3 for 11C-MeAIB, arrows) and left hilar ln (SUVmax = 21.9 for 18F-FDG, 11.5 for 11C-MeAIB, arrowheads). It should be noted that 18F-FDG uptake was relatively greater in the periphery than that of 11C-MeAIB. It was confirmed as concomitant inflammatory changes in the tissue surrounding the lesion. A small bone metastasis was also suspected in the right scapula (not pathologically confirmed)
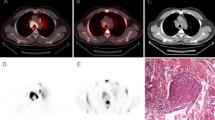
A case of sarcoidosis. Forty-four-year-old asymptomatic female patient. She had been followed as an outpatient after complete remission (more than 1 year previously) of malignant abdominal lymphoma (post-chemotherapy). She had no history of sarcoidosis or uveitis. The patient underwent chest CT scan (a), which revealed multiple nodules (arrows) in the middle mediastinum. 18F-FDG PET (b: MIP image) showed high accumulation in these lesions (SUVmax = 6.0 for the highest uptake in right hilar lesion), but there was no other lesions in the body. 11C-MeAIB PET (c: MIP image) showed no accumulation in these lesions (SUVmax = 1.8, with the highest uptake in the mediastinum). Thoracoscopic biopsy revealed sarcoidosis (d: low magnification, e: high magnification)
A case of sarcoidosis with lung nodule and mediastinal lesions. Sixty-three-year-old asymptomatic male patient. He had been followed as an outpatient for mediastinal sarcoidosis in another hospital. A new lung nodule was detected on CT scan and he was referred to our hospital. Both 18F-FDG PET/CT and 11C-MeAIB PET/CT were performed (a, d: MIP image). There was high 18F-FDG accumulation (SUVmax = 12.3) in the right lower lobe lesion (b: CT, c: PET/CT image), multiple mediastinal lesions, including right hilar lymph nodes (SUVmax = 7.6, arrowheads) and also in the upper abdominal lymph nodes (cardiac involvement was also indicated and confirmed later). 11C-MeAIB PET/CT showed very low accumulation (SUVmax = 1.7) in the right lower lobe lesion (e: CT, f: PET/CT image) (SUVmax = 1.7), multiple mediastinal lesions, including left hilar lymph nodes (SUVmax = 1.7, arrowheads), and also in the upper abdominal lymph nodes. Follow-up CT scan revealed shrinkage of lung nodule, and the diagnosis of sarcoidosis was confirmed
A case of metastatic colon cancer. Sixty-two year-old asymptomatic female patient and without elevated tumor markers. She had been followed as an outpatient after the resection of primary advanced colon adenocarcinoma with mucin secretion. In the follow-up period (1 year after surgery), the patient underwent a contrast-enhanced chest CT scan (a), which revealed a small lung nodule in the left apex. Both 18FDG and 11C-MeAIB PET showed faint accumulation in the left upper lobe lesion (SUVmax = 3.12 for 18F-FDG, 2.06 for 11C-MeAIB). Both 18F-FDG and 11C-MeAIB PET diagnoses were “equivocal” (visual diagnosis: negative, quantitative diagnosis: positive) (b, c). After close follow-up by CT scan and tumor markers, she underwent left upper lobe resection, and this lesion was confirmed as metastatic colon cancer with mucin secretion by pathology (d: low magnification, e: high magnification)
Semi-quantitative analysis of 18FDG uptake and 11C-MeAIB uptake
The average SUVmax and SUVmean for 18F-FDG and 11C-MeAIB uptake in normal lung and mediastinum are shown in Table 2. The average SUVmax of 18F-FDG in malignant lesions was significantly higher than that in benign lesions for 42 pulmonary nodules, while not significantly different for 38 benign and malignant mediastinal lesions. There was a wide overlap in 18F-FDG uptake between malignant and benign chest diseases, resulting in many false positive cases on 18F-FDG PET/CT, especially in mediastinal lesions.
The average SUVmax of malignant lesions with 11C-MeAIB PET/CT was significantly lower than that for 18F-FDG PET/CT. On the other hand, 11C-MeAIB uptake by malignant and benign lesions showed greater statistical differences both among pulmonary nodules and mediastinal lesions.
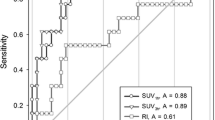
Figure 5 shows the result of ROC analyses for 18F-FDG and 11C-MeAIB, in which the diagnostic accuracies were obtained from the SUVmax values of each tumor in both PET/CT studies. An SUVmax = 3.0 was used as the threshold for 18F-FDG diagnosis. Other thresholds (SUVmax = 2.0, 2.5, 3.5, 4.0) gave similar or worse diagnostic results. In 11C-MeAIB PET/CT studies, an SUVmax = 2.0 was used as the optimum threshold. 11C-MeAIB scans showed a higher value than 18F-FDG scans both in patient-based (Fig. 5) and lesion-based diagnoses (not shown).
Receiver operating characteristic curve (ROC) analyses for the diagnostic accuracy of 11C-MeAIB and 18F-FDG using semi-quantitative analysis. The area under the curve (AUC) value for 11C-MeAIB PET/CT was 0.85 with standard error: 0.039, 95 % CI: 0.77–0.92 and p < 0.0001. AUC for 18F-FDG PET/CT was 0.70 with standard error: 0.056, 95 % CI: 0.60–0.82 and p < 0.001. These analyses indicated the better diagnostic accuracy of 11C-MeAIB for chest diseases (p < 0.05)
Patient-based diagnostic results
Visual diagnosis of 18FDG and 11C-MeAIB on a per-patient basis in 59 cases is shown in Table 3 (top). The accuracy of 18FDG was 72.9 %, which was better than that from semi-quantitative analysis. Although 18FDG uptake was often equivocally positive, the final diagnosis was judged as true negative in many benign or inflammatory cases because of the analysis of pattern and location of the 18F-FDG uptake. Fifteen false positive cases with 18F-FDG were as follows: granulomatous inflammatory lung nodules and mediastinal lymphadenopathy confirmed by surgical resection (n = 6), mediastinal sarcoid lymphadenopathy by lymph node biopsy (n = 5), mediastinal IgG4-related lymphadenopathy by lymph node biopsy (n = 1), and non-specific inflammatory change followed for more than 12 months (n = 3). The accuracy of 11C-MeAIB was 81.4 %. Ten false positive cases with 11C-MeAIB were as follows: granulomatous inflammatory lung nodules and mediastinal lymphadenopathy confirmed by surgical resection (n = 4), mediastinal sarcoid lymphadenopathy by lymph node biopsy (n = 3), mediastinal IgG4-related lymphadenopathy by lymph node biopsy (n = 1), and non-specific inflammatory change followed for more than 12 months (n = 2). Nine of these 11C-MeAIB false positive cases were also false positive with 18F-FDG. One false negative case was of metastatic colon cancer, which was also false negative in 18F-FDG PET images (Fig. 4).
In the semi-quantitative diagnoses (59 cases) with SUVmax = 3.0 cut-off value, the accuracy of 18F-FDG PET/CT was 47.5 %, in which there were many false positive results as compared with those at visual diagnosis (Table 3 bottom). There were two false negative cases with 18F-FDG PET/CT; both were papillary adenocarcinomas with ground-glass opacity (n = 2, diameter: 25 and 25 mm, SUVmax: 1.89 and 2.00). The accuracy of 11C-MeAIB PET/CT with SUVmax = 2.0 cut-off value was 74.6 %. There were 13 false positive cases and 2 false negative cases. The false positive cases were as follows: granulomatous inflammatory mediastinal lymphadenopathy confirmed by surgical resection (n = 4), mediastinal sarcoid lymphadenopathy by lymph node biopsy (n = 3), IgG4-related syndrome (n = 1), and non-specific inflammatory change followed for more than 12 months (n = 5). The two false negative cases were papillary adenocarcinomas with ground-glass opacity (n = 2, diameter: 25 and 30 mm, SUVmax: 1.74 and 1.93). The former one was also false negative case with 18FDG, but the latter one was true positive with 18FDG.
Lesion-based semi-quantitative diagnostic results
In the differential diagnosis of pulmonary nodules with SUVmax = 3.0 cut-off value, the accuracy of 18F-FDG PET/CT was 65.0 % (Table 4 top). The accuracy of 18F-FDG PET/CT for the diagnosis of mediastinal nodules (Table 4 bottom) was only 31.6 %. There were 26 cases of false positive results with 18F-FDG PET/CT, and the positive predictive value was only 27.8 %. 11C-MeAIB PET/CT showed better accuracy. The accuracy of 11C-MeAIB with SUVmax = 2.0 cut-off value for pulmonary nodules and mediastinal nodules was 76.2 and 76.3 %, respectively. In mediastinal nodules, there were only nine cases of false positive results, for a positive predictive value of 52.6 %.
Discussion
Several amino acid compounds have been suggested as feasible candidates for oncologic PET tracers which can overcome the drawbacks of 18F-FDG. 11C-MeAIB is considered to be one of the most promising amino acid radiotracers in clinical oncology. To our knowledge, the present study is the first to evaluate the clinical application of 11C-MeAIB PET-to-chest lesion diagnosis.
Our principal finding is that the diagnostic results of 11C-MeAIB PET/CT were better than those of 18F-FDG PET/CT, especially for the identification of non-malignant lesions. Table 3 clearly reveals the higher specificity of 11C-MeAIB PET/CT in these cases. In the evaluation of mediastinal lesions, 11C-MeAIB PET/CT showed 67.9 % specificity in lesion-based semi-quantitative diagnosis, while 18F-FDG PET/CT’s specificity was only 7.1 % (Table 4). The low positive predictive value (27.8 %) of 18F-FDG PET/CT confirms that positive uptake of 18F-FDG is not reliably diagnostic of malignancy. We believe that 11C-MeAIB PET/CT would make a great contribution in the diagnosis of patients with pulmonary nodules or mediastinal lesions, when CT and 18F-FDG PET/CT shows equivocal findings.
It should be noted that 11C-MeAIB PET/CT displayed high diagnostic accuracy in the evaluation of sarcoidosis. There were 20 sarcoid lesions in 14 patients and the average lesion 18F-FDG SUVmax was 7.3 ± 4.5, while that of 11C-MeAIB was 1.8 ± 0.7 (Table 5). Visual diagnosis with 18F-FDG PET/CT showed false positives in six patients, while those with 11C-MeAIB PET/CT showed false positives in three patients. Although 18F-FDG PET/CT can diagnose sarcoidosis by the specific uptake pattern of hilar and mediastinal lesions, sarcoidosis can form pulmonary nodules as well, and those lesions can be difficult to distinguish from malignancies. In addition, malignancy is often observed synchronously or metachronously in patients with sarcoidosis. In the present study, there were five pulmonary lesions (all of them finally confirmed as benign) in five patients with sarcoidosis. In these cases, 11C-MeAIB PET/CT played a useful diagnostic role (Fig. 3). Since 11C-MET accumulates in sarcoidosis [14, 15], it is suggested that 11C-MeAIB may be superior to 11C-MET in the differentiation of sarcoidosis from malignancy. Our result is compatible with previous studies using other amino acid PET tracers, such as [18F]-methyltyrosine (18F-FMT) [21]. Kaira et al. suggested in their report that the use of 18F-FMT PET in combination with 18F-FDG PET may be effective for this purpose. In terms of biological mechanism, it is not fully understood why 11C-MET and the other amino acid PET tracers (11C-MeAIB and 18F-FMT) show different uptake patterns in sarcoidosis. One of the conceivable mechanisms for the low uptake in sarcoidosis lesions of 11C-MeAIB and 18FMT is that these PET tracers, as artificial amino acids, are not metabolized in vivo [16, 22]. Concerning in vivo instability of 11C-MET, inflammatory lesion can be misdiagnosed by 11C-MET PET because of its non-specific accumulation of free 11C in blood when an inflammatory lesion shows hypervascularity. Comparative study of these amino acid PET tracers should be further evaluated.
In the diagnosis of malignancy, the sensitivity of 18F-FDG and 11C-MeAIB based on semi-quantitative patient-based diagnosis showed the same values (90.9 %) (Table 4). In addition, the uptake of 11C-MeAIB correlated well with 18F-FDG uptake and there were basically no discrepant cases (Fig. 6). 18F-FDG SUVs in malignant cases was usually two to three times higher than those of 11C. In previous studies using 11C-MET and 18F-FMT, 11C-MET and 18F-FMT SUVs were also two to three times lower than those of 18FDG [21, 23–25]. This may be a common drawback of amino acid PET tracers. Although our group included several different types of lung cancers, such as adenocarcinoma, squamous cell carcinoma, and small cell carcinoma, there was no significant difference in the uptake intensity of 11C-MeAIB among the different histological types. It is not what we anticipated for 11C-MeAIB PET/CT’s use as a predictor of therapeutic effect, because amino acid transporters are known to work as carriers of chemotherapeutic agents, such as cisplatin, methotrexate, taxol, and melphalan [26–28]. The role of 11C-MeAIB PET/CT as an imaging modality for patient-tailored medicine is unknown. Further study of pre- and post-chemotherapeutic 11C-MeAIB PET or PET/CT is needed.
Relationship between SUVmax of 11C-MeAIB and that of 18F-FDG of each lesion in both PET study using Logistic regression. In malignant lesions, SUVmax of 11C-MeAIB shows a significant linear relationship with that of 18F-FDG (p < 0.0001, R 2 = 0.406). On the other hand, that of 11C-MeAIB also shows a linear correlation with that of 18F-FDG but not significant in benign lesions (p = 0.055, R 2 = 0.078)
Another drawback of 11C-MeAIB is its high physiological uptake by liver. It means that 11C-MeAIB PET/CT cannot be performed as a first-choice diagnostic modality in the evaluation of chest malignancies, because liver metastasis is common in lung cancer. Therefore, 11C-MeAIB PET or PET/CT cannot be performed as a study for staging of advanced lung cancer. This is why we focused our study only on the differential diagnosis in chest diseases, and excluded cases with apparent distant metastasis and direct invasion of neighboring organs.
Conclusions
11C-MeAIB PET/CT was useful in the differential diagnosis of pulmonary and mediastinal mass lesions found on CT. 11C-MeAIB PET or PET/CT showed higher specificity than that of 18F-FDG PET/CT in differentiating between benign and malignant disease. Our data suggest that the combination of 18F-FDG and 11C-MeAIB may improve the evaluation of chest lesions, when CT and 18F-FDG PET/CT are equivocal.
References
Poeppel TD, Krause BJ, Heusner TA, Boy C, Bockisch A, Antoch G. PET/CT for the staging and follow-up of patients with malignancies. Eur J Radiol. 2009;70(3):382–92.
Metser U, Even-Sapir E. Increased (18)F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions found on whole-body positron emission tomography/computed tomography (PET/CT): accumulated data from four years of experience with PET/CT. Semin Nucl Med. 2007;37(3):206–22.
Higashi T, Saga T, Nakamoto Y, Ishimori T, Fujimoto K, Doi R, Imamura M, Konishi J. Diagnosis of pancreatic cancer using fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET)—usefulness and limitations in “clinical reality”. Ann Nucl Med. 2003;17(4):261–79 (review).
Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics. 1999;19(1):61–77 (review).
Chang JM, Lee HJ, Goo JM, Lee HY, Lee JJ, Chung JK, Im JG. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J Radiol. 2006;7:57–69 (review).
Hu M, Han A, Xing L, Yang W, Fu Z, Huang C, et al. Value of dual-time-point FDG PET/CT for mediastinal nodal staging in non-small-cell lung cancer patients with lung comorbidity. Clin Nucl Med. 2011;36:429–33.
Deppen S, Putnam JB Jr, Andrade G, Speroff T, Nesbitt JC, Lambright ES, et al. Accuracy of FDG-PET to diagnose lung cancer in a region of endemic granulomatous disease. Ann Thorac Surg. 2011;92:428–32.
Lee JW, Kim BS, Lee DS, Chung JK, Lee MC, Kim S, et al. 18F-FDG PET/CT in mediastinal lymph node staging of non-small-cell lung cancer in a tuberculosis-endemic country: consideration of lymph node calcification and distribution pattern to improve specificity. Eur J Nucl Med Mol Imaging. 2009;36:1794–802.
Antoni G, Långström B. Radiopharmaceuticals: molecular imaging using positron emission tomography. Handb Exp Pharmacol. 2008;(185 Pt 1):177–201. Review.
Wadsak W, Mitterhauser M. Basics and principles of radiopharmaceuticals for PET/CT. Eur J Radiol. 2010;73(3):461–9 (review).
Jager PL, Vaalburg W, Pruim J, de Vries EG, Langen KJ, Piers DA. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med. 2001;42(3):432–45 (review).
Leskinen-Kallio S, Ruotsalainen U, Någren K, Teräs M, Joensuu H. Uptake of carbon-11-methionine and fluorodeoxyglucose in non-Hodgkin’s lymphoma: a PET study. J Nucl Med. 1991;32(6):1211–8.
Fujibayashi Y, Kawai K, Yonekura Y, Matsumoto K, Konishi J, Yokoyama A. Problems of [S-methyl-11C]-l-methionine as a protein synthesis marker in the pancreas. Ann Nucl Med. 1990;4(1):29–33.
van Waarde A, Jager PL, Ishiwata K, Dierckx RA, Elsinga PH. Comparison of sigma-ligands and metabolic PET tracers for differentiating tumor from inflammation. J Nucl Med. 2006;47(1):150–4.
Yamada Y, Uchida Y, Tatsumi K, Yamaguchi T, Kimura H, Kitahara H, et al. Fluorine-18-fluorodeoxyglucose and carbon-11-methionine evaluation of lymphadenopathy in sarcoidosis. J Nucl Med. 1998;39(7):1160–6.
Sutinen E, Jyrkkiö S, Grönroos T, Haaparanta M, Lehikoinen P, Någren K. Biodistribution of [11C] methylaminoisobutyric acid, a tracer for PET studies on system A amino acid transport in vivo. Eur J Nucl Med. 2001;28(7):847–54.
Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70(1):43–77 (review).
Asola MR, Virtanen KA, Peltoniemi P, Någren K, Yu M, Mattila K, et al. Amino acid uptake in the skeletal muscle measured using [11C]methylaminoisobutyrate (MEAIB) and PET. Eur J Nucl Med Mol Imaging. 2002;29(11):1485–91.
Sutinen E, Jyrkkiö S, Alanen K, Någren K, Minn H. Uptake of [N-methyl-11C]alpha-methylaminoisobutyric acid in untreated head and neck cancer studied by PET. Eur J Nucl Med Mol Imaging. 2003;30(1):72–7.
Någren K, Sutinen E, Jyrkkiö S. [N-methyl-11C]MeAIB, a tracer for system A amino acid transport: preparation from [11C]Methyl Triflate and HPLC metabolite analysis of plasma samples after intravenous administration in man. J Label Cpd Radiopharm. 2000;43:1013–21.
Kaira K, Oriuchi N, Otani Y, Yanagitani N, Sunada N, Hisada T, et al. Diagnostic usefulness of fluorine-18-alpha-methyltyrosine positron emission tomography in combination with 18F-fluorodeoxyglucose in sarcoidosis patients. Chest. 2007;131:1019–27.
Inoue T, Tomiyoshi K, Higuchi T, Ahmed K, Sarwar M, Woyagi K, et al. Biodistribution studies on L-[3-18F] fluoro-alpha-methyltyrosine: a potential tumor-detecting agent. J Nucl Med. 1998;39:663–7.
Kanegae K, Nakano I, Kimura K, Kaji H, Kuge Y, Shiga T, et al. Comparison of MET-PET and FDG-PET for differentiation between benign lesions and lung cancer in pneumoconiosis. Ann Nucl Med. 2007;21(6):331–7.
Sasaki M, Kuwabara Y, Yoshida T, Nakagawa M, Koga H, Hayashi K, et al. Comparison of MET-PET and FDG-PET for differentiation between benign lesions and malignant tumors of the lung. Ann Nucl Med. 2001;15(5):425–31.
Hsieh HJ, Lin SH, Lin KH, Lee CY, Chang CP, Wang SJ. The feasibility of 11C-methionine-PET in diagnosis of solitary lung nodules/masses when compared with 18F-FDG-PET. Ann Nucl Med. 2008;22(6):533–8.
Kashani-Sabet M, Scanlon KJ. Drug selectivity and membrane transport properties of normal human lymphocytes. Chemotherapy. 1989;35(5):363–72.
del Amo EM, Urtti A, Yliperttula M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci. 2008;35(3):161–74.
Wu Y, Shen D, Chen Z, Clayton S, Vadgama JV. Taxol induced apoptosis regulates amino acid transport in breast cancer cells. Apoptosis. 2007;12(3):593–612.
Acknowledgments
This study was supported by two categories of the Grants-in-Aid for Scientific Research programmed by the Japan Society for the Promotion of Science (JSPS), Challenging Exploratory Research (Grant #50324629, 2009–2010), and Scientific Research (B) (Grant #23300360, 2011–2013).
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Nishii, R., Higashi, T., Kagawa, S. et al. Diagnostic usefulness of an amino acid tracer, α-[N-methyl-11C]-methylaminoisobutyric acid (11C-MeAIB), in the PET diagnosis of chest malignancies. Ann Nucl Med 27, 808–821 (2013). https://doi.org/10.1007/s12149-013-0750-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-013-0750-4