Abstract
Objectives
Semiquantitative evaluation of tracer uptake in basal ganglia is superior to visual assessment of images in dopamine transporter (DAT) scintigraphy especially in follow-up of the patients. Manual drawing of regions of interest (ROIs) in two-dimensional (2D) transaxial slices of the single photon emission computed tomography (SPECT) datasets leads to a large inter- and intra-reader variability, while being time consuming. Our aim was to investigate a technique that extracts 3D ROIs in a fully automated fashion and thus might provide reproducible user-independent results allowing better follow-up control and large-scale clinical studies.
Methods
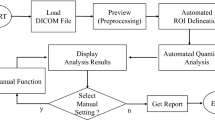
The highest activity of 123IFP-CIT is expected in the basal ganglia. The proposed method (Spectalyzer) uses the following steps to localize this maximum and extract the ROIs in 3D: (1) Dithers the SPECT volume to obtain a 3D volume with binary only. (2) Models the obtained point distributions as two multivariate Gaussian distributions and estimated their parameters using the expectation maximization algorithm. (3) Using the original SPECT activity values, thresholding is performed using a fixed percentage of maximum activity as a parameter to obtain the 3D ROIs. (4) A reference volume in the occipital region is automatically found based on the location of the two ROIs. (5) From the 3D ROIs, statistical information like mean and median activity and the volume is extracted, relative to the activity in the reference region. The resulting values are compared with values from manual 2D ROIs. Further validation is performed by means of an anthropomorphic striatal phantom.
Results
The method was evaluated on 12 SPECT volumes including anthropomorphic striatal phantoms. In all cases the two basal-ganglia were successfully localized and the 3D ROIs estimated, with perfect reproducibility. The obtained values for the mean activity showed the same trend with the values obtained manually and also with the results of the 2D semiautomatic software, but without the substantial inter- and intra-reader variations.
Conclusions
The proposed method is successful in finding the 3D ROIs and performing the subsequent measurements automatically. It is proposed as an automatic reproducible approach for semiquantitative analysis of DAT scintigraphy.



Similar content being viewed by others
References
Booij J, Speelman JD, Horstink MW, Wolters EC. The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPECT in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonisms. Eur J Nucl Med. 2001;28:266–72.
Van Laere K, Everaert L, Lieven A, Gonce M, Vandenberghe W, Vander Borght T. The cost effectiveness of 123I-FP-CIT SPECT imaging in patients with an uncertain clinical diagnosis of parkinsonism. Eur J Nucl Med. 2008;35:1367–76.
Booij J, Tissingh G, Winogrodzka A, vam Royen EA. Imaging of the dopaminergic neurotransmission system using single-photon emission tomography and positron emission tomography in patients with parkinsonism [review]. Eur J Nucl Med. 1999;26:171–82.
Marshall V, Grosset D. Role of dopamine transporter imaging in routine clinical practice [review]. Mov Disord. 2003;18:1415–23.
Wolters EC, Francot C, Bergmans P, Winogrozka A, Booij J, Berendse HW, et al. Preclinical (premotor) Parkinson’s disease. J Neurol. 2000;247(Suppl 2):II103–9.
Walker Z, Costa DC, Walker RW, Shaw K, Gacinovic S, Stevens T, et al. Differentiation of dementia with Lewy bodies from Alzheimer’s disease using a dopaminergic presynaptic ligand. J Neurol Neurosurg Psychiatry. 2002;73:134–40.
McKeith I, O’Brian J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123-I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6:305–13.
Tatsch K, Asenbaum S, Bartenstein P, Catafau A, Halldin C, Pillowsky LS, et al. European Association of Nuclear Medicine procedure guidelines for brain neurotransmission SPET using 123I-labelled dopamine transporter ligands. Eur J Nucl Med. 2002;BP29:30–5.
Cot A, Falcón C, Crespo C, Sempau J, Pareto D, Bullich S, et al. Absolute quantification in dopaminergic neurotransmission SPECT using a Monte Carlo-based scatter correction and fully 3-dimensional reconstruction. J Nucl Med. 2005;46:1497–504.
Beekman FJ, de Jong HWAM, Geloven S. Efficient fully 3-D iterative SPECT reconstruction with Monte Carlo-based scatter compensation. IEEE Trans Med Imaging. 2002;21:867–77.
Colloby SJ, Williams ED, Burn DJ, Lloyd JJ, McKeith IG, O’Brien JT. Progression of dopaminergic degeneration in dementia with Lewy bodies and Parkinson’s disease with and without dementia assessed using 123I-FP-CIT SPECT. Eur J Nucl Med Mol Imaging. 2005;32:1176–85.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirzaei, S., Zakavi, R., Rodrigues, M. et al. Fully automated 3D basal ganglia activity measurement in dopamine transporter scintigraphy (Spectalyzer). Ann Nucl Med 24, 295–300 (2010). https://doi.org/10.1007/s12149-010-0353-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-010-0353-2