Abstract
Objective
To observe the antitumor effect of 131I-17-allylamino-17-demethoxygeldanamycin (131I-17-AAG) in vitro/in vivo and explore its antitumor mechanism with a view to its potential therapeutic application.
Methods
131I-17-AAG was prepared by the reaction of 17-AAG with Na [131I] in the presence of hydrogen peroxide. The effects of 13117-AAG on cell growth inhibition and cell cycle distribution in vitro were studied in BEL-7402 cells lines. Following BEL-7402 tumor implantation by subcutaneous xenografts into nude mice, the reagents were injected through the tail vein, and the tumor volume was measured and analyzed. At the end of the experiment, tumor specimens were processed for histopathological analysis. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) was used to investigate apoptosis. The expression change of Akt2 was tested by Western-blot analysis.
Results
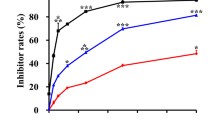
Methyl-thiazolyl-tetrazolium assay showed inhibition rates of 27.7 ± 5.3%, 57.3 ± 4.3%, and 63.7 ± 3.1%, in Na131I group, 17-AAG group, and 131I-17-AAG group, respectively. The inhibition rate in the 131I-17-AAG group differed significantly between Na131I group and 17-AAG group (F = 229.49, P < 0.001). Following 48 h of treatment with the drug in each group, flow cytometry analysis indicated that detected sub-G peaks (black) were 1.54 ± 0.13%, 5.72 ± 1.05%, 12.97 ± 1.44%, and 20.65 ± 1.36%, in dimethyl sulfoxide (DMSO) group, Na131I group, 17-AAG group, and 131I-17-AAG group, respectively. Following infusion for 32 days, the tumor volumes in the 131I-17-AAG group were significantly smaller than those in the DMSO group (F = 24.18, P < 0.001) or the 131I group (F = 20.68, P < 0.001). Histopathological and TUNEL analyses showed that 131I-17-AAG inhibited the proliferation of tumor cells and induced apoptosis. The expression of Akt2 in 131I-17-AAG was significantly lower than that in the DMSO group or 131I group.
Conclusions
131I-17-AAG can effectively inhibit the growth of BEL-7402 tumor cells in vitro and in vivo. 131I-17-AAG is a promising agent for the treatment of BEL-7402 cell tumor.
Similar content being viewed by others
References
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70.
Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa): an inhibitor of EGFR tyrosine kinases. Clin Cancer Res 2000;6:4885–4892.
Omura S, Iwai Y, Takahashi Y, Sadakane N, Nakagawa A, Oiwa H, et al. Herbimycin: a new antibiotic produced by a strain of Streptomyces. J Antibiot (Tokyo) 1979;32:255–261.
Uehara Y, Hori M, Takeuchi T, Umezawa H. Screening of agents which convert ‘transformed morphology’ of Rous sarcoma virus-infected rat kidney cells to ‘normal morphology: identification of an active agent as herbimycin and its inhibition of intracellular src kinase. Jpn J Cancer Res 1985; 76:672–675.
Uehara Y, Hori M, Takeuchi T, Umezawa H. Phenotypic change from transformed to normal induced by benzoquinonoid ansamycins accompanies inactivation of p60src in rat kidney cells infected with Rous sarcoma virus. Mol Cell Biol 1986;6:2198–2206.
Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell 1997;89:239–250.
Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA 1994;91:8324–8328.
Uehara Y, Murakami Y, Mizuno S, Kawai S. Inhibition of transforming activity of tyrosin kinase oncogenes by herbimycin A. Virology 1988;164:294–298.
Webb CP, Hose CD, Koochekpour S, Jeffers M, Oskarsson M, Sausville E, et al. The geldanamycins are potent inhibitors of the hepatocyte growth factor/scatter factor-met-urokinase plasminogen activator-plasmin proteolytic network. Cancer Res 2000;60:342–349.
Xu W, Yuan X, Jung YJ, Yang Y, Basso A, Rosen N, et al. The heat shock protein 90 inhibitor geldanamycin and the ErbB inhibitor ZD1839 promote rapid PP1 phosphatasedependent inactivation of AKT2 in ErbB2 over expressing breast cancer cells. Cancer Res 2003;63:7777–7784.
Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein Hsp90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA 1994;91:8324–8328.
Grenert JP, Sullivan WP, Fadden P, Haystead TA, Clark J, Mimnaugh E, et al. The amino-terminal domain of heat shock protein 90 (hsp900) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem 1997;272:23843–23850.
Whitesell L, Sutphin P, An WG, Schulte T, Blagosklonny MV, Neckers L. Geldanamycin-stimulated destabilization of mutated p53 is mediated by proteasome in vivo. Oncogene 1997;14:2809–2816.
Hostein I, Robertson D, Di Stefano F, Workman P, Clarke PA. Inhibition of signal transduction by the Hsp90 inhibitor 17-allyl-amino-17-demethoxy geldanamycin results in cytostasis and apoptosis. Cancer Res 2001;61:4003–4009.
Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, et al. A high affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 2003;425: 407–410.
Yang J, Yang JM, Iannone M, Shih WJ, Lin Y, Hait WN. Disruption of the EF-2kinase/Hsp90 protein complex: a possible mechanism to inhibit glioblastoma by geldanamycin. Cancer Res 2001;61:4010–4016.
Amin K, Ip C, Jimenez L, Tyson C, Behrsing H. In vitro detection of differential and cell-specific hepatobiliary toxicity induced by geldanamycin and 17-allylamino geldanamycin using dog liver slices. Toxicol Sci 2005;87:442–450.
Clamp AR, Schöffski P, Valle JW, Wilson RH, Marreaud S, Govaerts AS, et al. A phase I and pharmacokinetic study of OSI-7904L: a liposomal thymidylate synthase inhibitor in combination with oxaliplatin in patients with advanced colorectal cancer. Cancer Chemother Pharmacol 2008;61: 579–585.
Zhang H, Chung D, Yang YC, Neely L, Tsurumoto S, Fan J, et al. Identification of new biomarkers for clinical trials of Hsp90 inhibitors. Mol Cancer Ther 2006;5:1256–1264.
Daozhen C, Lu L, Min Y, Xinyu J, Ying H. Synthesis of 131I-labeled-[131I] iodo-17-allylamino-17-demethoxy geldanamycin ([131I]Iodo-17-AAG) and its biodistribution in mice. Cancer Biother Radiopharm 2007;22:607–612.
Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutics: an update. Expert Opin Emerg Drugs 2005;10:137–149.
Goetz MP, Toft DO, Ames MM, Erlichman C. The Hsp90 chaperone complex as a novel target for cancer therapy. Ann Oncol 2003;14:1169–1176.
Hostein I, Robertson D, DiStefano F, Workman P, Clarke PA. Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res 2001;61:4003–4009.
Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 1997;90:65–75.
Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N. Inhibition of heat shock protein 90 function down-regulates Akt21 kinase and sensitizes tumors to Taxol. Cancer Res 2003;63: 2139–2144.
Doong H, Rizzom K, Fangm S, Kulpam V, Weissmanm AM, Kohn EC. CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complex-mediated protein degradation: accumulation of poly-ubiquitinated Hsp90 client proteins. J Biol Chem 2003;278:28490–28500.
Workman P. Combinatorial attack on multistep oncogenesis by inhibiting the Hsp90 molecular chaperone. Cancer Lett 2004;206:149–157.
Calabrese C, Frank A, Maclean K, Gilbertson R. Medulloblastoma sensitivity to 17-allylamino-17-demethoxygeldana mycin requires MEK/ERKM. J Biol Chem 2003;278: 24951–24959.
Georgakis GV, Li Y, Younes A. The heat shock protein 90 inhibitor 17-AAG induces cell cycle arrest and apoptosis in mantle cell lymphoma cell lines by depleting cyclin D1, Akt, Bid and activating caspase 9. Br J Haematol 2006;135:68–71.
Macklis RM, Lin JY, Beresford B, Atcher RW, Hines JJ, Humm JL. Cellular kinetics, dosimetry and radiobiology of α-particle radioimmunotherapy: introduction of apoptosis. Radiat Res 1992;130:220–226.
Russell J, Burgan W, Oswald K, Camphausen K, Tofilon P. Enhanced cell killing induced by the combination of radiation and the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin: a multitarget approach to radiosensitization. Clin Cancer Res 2003;9:3749–3755.
Harashima K, Akimoto T, Nonaka T, Tsuzuki K, Mitsuhashi N, Nakano T. Heat shock protein 90 (Hsp90) chaperone complex inhibitor, radicicol, potentiated radiation-induced cell killing in a hormone-sensitive prostate cancer cell line through degradation of the androgen receptor. Int J Radiat Biol 2005;81:63–76.
Kobayashi S, Nantz R, Kitamura T, Higashikubo R, Horikoshi N. Combined inhibition of extracellular signalregulated kinases and HSP90 sensitizes human colon carcinoma cells to ionizing radiation. Oncogene 2005;24:3011–3019.
Schulte TW, Neckers LM. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother Pharmacol 1998;42:273–279.
Kelland LR, Sharp SY, Rogers PM, Myers TG, Workman P. DT-diaphorase expression and tumor cell sensitivity to 17-allylamino,17-demethoxygeldanamycin, an inhibitor of heat shock protein 90. J Natl Cancer Inst 1999;91:1940–1949.
Chiosis G, Huezo H, Rosen N, Mimnaugh E, Whitesell L, Neckers L. 17AAG: low target binding affinity and potent cell activity-finding an explanation. Mol Cancer Ther 2003;2: 123–129.
Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res 2003;159: 283–300.
Kumar P, Miller AI, Polverini PJ. P38 MAPK mediates gamma-irradiation-induced endothelial cell apoptosis, and vascular endothelial growth factor protects endothelial cells through the phosphoinositide 3-kinase-Akt2-Bcl-2 pathway. J Biol Chem 2004;279:43352–43360.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wenyong, T., Lu, L., Daozhen, C. et al. An experimental study on the antitumor effect of 131I-17-AAG in vitro and in vivo. Ann Nucl Med 23, 113–122 (2009). https://doi.org/10.1007/s12149-008-0215-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-008-0215-3