Abstract
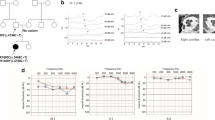
Pathogenic variants in COCH, encoding cochlin, cause DFNA9 deafness disorder with characteristic histopathologic findings of cochlin deposits in the inner and middle ears. Here, we present the first case of deafness associated with bilateral external auditory canal (EAC) cochlin deposits, previously unreported evidence suggestive of cochlin-derived amyloid formation, and a novel COCH variant. A 54-year-old woman presented with progressive sensorineural hearing loss and bilateral EAC narrowing by subcutaneous thickening. Excision and histologic evaluation of tissue from both EACs showed paucicellular eosinophilic aggregates containing multiple Congo red-positive foci with yellow and green birefringence under crossed polarization light microscopy. Mass spectrometry performed on both the Congo red-positive and Congo red-negative areas identified cochlin as the most abundant protein, as well as a low abundance of universal amyloid signature peptides only in the Congo red-positive areas. Peptides indicative of a canonical amyloid type were not detected. Electron microscopy showed haphazard, branched microfibrils (3–7 nm in diameter) consistent with cochlin, as well as swirling fibrils (10–24 nm in diameter) reminiscent of amyloid fibrils. Cochlin immunohistochemical staining showed positivity throughout the aggregates. Sequencing of the entire COCH gene coding region from the patient’s blood revealed a novel variant resulting in a non-conservative amino acid substitution of isoleucine to phenylalanine (c.1621A>T, p.I541F) in the vWFA2 domain at the protein’s C-terminus. Our findings reveal a new pathologic manifestation of cochlin, raise the possibility of previously undescribed cochlin-derived amyloid formation, and highlight the importance of thoroughly investigating all aggregative tissue findings in the practice of diagnostic pathology.







Similar content being viewed by others
References
Robertson NG, Cremers CW, Huygen PL, Ikezono T, Krastins B, Kremer H, et al. Cochlin immunostaining of inner ear pathologic deposits and proteomic analysis in DFNA9 deafness and vestibular dysfunction. Hum Mol Genet. 2006;15(7):1071–85. https://doi.org/10.1093/hmg/ddl022.
Ikezono T, Omori A, Ichinose S, Pawankar R, Watanabe A, Yagi T. Identification of the protein product of the Coch gene (hereditary deafness gene) as the major component of bovine inner ear protein. Biochem Biophys Acta. 2001;1535(3):258–65.
Robertson NG, O’Malley JT, Ong CA, Giersch AB, Shen J, Stankovic KM, et al. Cochlin in normal middle ear and abnormal middle ear deposits in DFNA9 and Coch (G88E/G88E) mice. J Assoc Res Otolaryngol. 2014;15(6):961–74. https://doi.org/10.1007/s10162-014-0481-9.
Robertson NG, Lu L, Heller S, Merchant SN, Eavey RD, McKenna M, et al. Mutations in a novel cochlear gene cause DFNA9, a human nonsyndromic deafness with vestibular dysfunction. Nat Genet. 1998;20(3):299–303. https://doi.org/10.1038/3118.
Khetarpal U, Schuknecht HF, Gacek RR, Holmes LB. Autosomal dominant sensorineural hearing loss. Pedigrees, audiologic findings, and temporal bone findings in two kindreds. Arch Otolaryngol-Head Neck Surg. 1991;117(9):1032–42.
McCall AA, Linthicum FH Jr, O’Malley JT, Adams JC, Merchant SN, Bassim MK, et al. Extralabyrinthine manifestations of DFNA9. J Assoc Res Otolaryngol. 2011;12(2):141–9. https://doi.org/10.1007/s10162-010-0245-0.
Robertson NG, Skvorak AB, Yin Y, Weremowicz S, Johnson KR, Kovatch KA, et al. Mapping and characterization of a novel cochlear gene in human and in mouse: a positional candidate gene for a deafness disorder, DFNA9. Genomics. 1997;46(3):345–54. https://doi.org/10.1006/geno.1997.5067.
Picciani R, Desai K, Guduric-Fuchs J, Cogliati T, Morton CC, Bhattacharya SK. Cochlin in the eye: functional implications. Prog Retin Eye Res. 2007;26(5):453–69. https://doi.org/10.1016/j.preteyeres.2007.06.002.
Py BF, Gonzalez SF, Long K, Kim MS, Kim YA, Zhu H, et al. Cochlin produced by follicular dendritic cells promotes antibacterial innate immunity. Immunity. 2013;38(5):1063–72. https://doi.org/10.1016/j.immuni.2013.01.015.
Nystrom A, Bornert O, Kuhl T, Gretzmeier C, Thriene K, Dengjel J, et al. Impaired lymphoid extracellular matrix impedes antibacterial immunity in epidermolysis bullosa. Proc Natl Acad Sci USA. 2018;115(4):E705-e14. https://doi.org/10.1073/pnas.1709111115.
Jung J, Yoo JE, Choe YH, Park SC, Lee HJ, Lee HJ, et al. Cleaved cochlin sequesters Pseudomonas aeruginosa and activates innate immunity in the inner ear. Cell Host Microbe. 2019;25(4):513-25.e6. https://doi.org/10.1016/j.chom.2019.02.001.
Bae SH, Robertson NG, Cho HJ, Morton CC, Jung DJ, Baek JI, et al. Identification of pathogenic mechanisms of COCH mutations, abolished cochlin secretion, and intracellular aggregate formation: genotype-phenotype correlations in DFNA9 deafness and vestibular disorder. Hum Mutat. 2014;35(12):1506–13. https://doi.org/10.1002/humu.22701.
Cho HJ, Park HJ, Trexler M, Venselaar H, Lee KY, Robertson NG, et al. A novel COCH mutation associated with autosomal dominant nonsyndromic hearing loss disrupts the structural stability of the vWFA2 domain. J Mol Med. 2012;90(11):1321–31. https://doi.org/10.1007/s00109-012-0911-2.
Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114(24):4957–9. https://doi.org/10.1182/blood-2009-07-230722.
Baudhuin LM, Lagerstedt SA, Klee EW, Fadra N, Oglesbee D, Ferber MJ. Confirming variants in next-generation sequencing panel testing by sanger sequencing. J Mol Diagn. 2015;17(4):456–61. https://doi.org/10.1016/j.jmoldx.2015.03.004.
Khetarpal U. DFNA9 is a progressive audiovestibular dysfunction with a microfibrillar deposit in the inner ear. The Laryngoscope. 2000;110(8):1379–84. https://doi.org/10.1097/00005537-200008000-00030.
Abuawad YG, Uchiyama J, Kakizaki P, Valente NYS. Primary cutaneous amyloidosis of the auricular concha—case report. An Bras Dermatol. 2017;92(3):433–4. https://doi.org/10.1590/abd1806-4841.20175864.
Alexander MP, Dasari S, Vrana JA, Riopel J, Valeri AM, Markowitz GS, et al. Congophilic fibrillary glomerulonephritis: a case series. Am J Kidney Dis. 2018;72(3):325–36. https://doi.org/10.1053/j.ajkd.2018.03.017.
Yakupova EI, Bobyleva LG, Vikhlyantsev IM, Bobylev AG. Congo red and amyloids: history and relationship. Biosci Rep. 2019. https://doi.org/10.1042/bsr20181415.
Howie AJ, Brewer DB. Optical properties of amyloid stained by Congo red: history and mechanisms. Micron. 2009;40(3):285–301. https://doi.org/10.1016/j.micron.2008.10.002.
Rocken C, Sletten K. Amyloid in surgical pathology. Virchows Arch. 2003;443(1):3–16. https://doi.org/10.1007/s00428-003-0834-y.
Street VA, Kallman JC, Robertson NG, Kuo SF, Morton CC, Phillips JO. A novel DFNA9 mutation in the vWFA2 domain of COCH alters a conserved cysteine residue and intrachain disulfide bond formation resulting in progressive hearing loss and site-specific vestibular and central oculomotor dysfunction. Am J Med Genet Part A. 2005;139A(2):86–95. https://doi.org/10.1002/ajmg.a.30980.
Acknowledgements
We acknowledge Mayo Clinic Clinical Tissue Proteomics Laboratory for performing mass spectrometry experiments.
Funding
C. C. Morton is supported by NIDCD R01DC015052 and the University of Manchester NIHR Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Basu, A., Boczek, N.J., Robertson, N.G. et al. First Report of Bilateral External Auditory Canal Cochlin Aggregates (“Cochlinomas”) with Multifocal Amyloid-Like Deposits, Associated with Sensorineural Hearing Loss and a Novel Genetic Variant in COCH Encoding Cochlin. Head and Neck Pathol 14, 808–816 (2020). https://doi.org/10.1007/s12105-019-01073-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-019-01073-7