Abstract
Soluble guanylate cyclase (sGC) is considered as the primary NO receptor across several known eukaryotes. The main interest regarding the biological role and its function, focuses on the H-NOX domain of the β1 subunit. This domain in its active form bears a ferrous b type heme as prosthetic group, which facilitates the binding of NO and other diatomic gases. The key point that still needs to be answered is how the protein selectively binds the NO and how the redox state of heme and coordination determines H-NOX active state upon binding of diatomic gases. H-NOX domain is present in the genomes of both prokaryotes and eukaryotes, either as a stand-alone protein domain or as a partner of a larger polypeptide. The biological functions of these signaling modules for a wide range of genomes, diverge considerably along with their ligand binding properties. In this direction, we examine the prokaryotic H-NOX protein domain from Nostoc punctiforme (Npun H-NOX). Herein, we first report the almost complete NMR backbone and side-chain resonance assignment (1H, 13C, 15 N) of Npun H-NOX domain together with the NMR chemical shift-based prediction of the domain’s secondary structure elements.
Similar content being viewed by others
Biological context
Soluble guanylate cyclase (sGC) is a widely known signaling molecule transducing signals mediated by the first messenger, a nitric oxide (NO). This NO sensor is largely found across the eukaryotic species, and it is responsible for vasodilation and neurotransmission in mammals (Papapetropoulos et al., 2015). Enzyme’s biological role and function focus on the H-NOX domain of the β1 subunit. The active form of H-NOX domain bears a heme molecule as natural ligand, which binds the NO and other diatomic gases. Upon binding of NO to the H-NOX domain of the sGC β1 subunit, sGC catalyses the conversion of guanosine 5-triphosphate (GTP) to the second sequential signal molecule, cyclic 3,5-guanosine monophosphate (cGMP) (Stone and Marletta, 1996). Disorders in its normal functioning or even the interruption of the signaling pathway result in a variety of pathological conditions (hypertension, strokes, erectile dysfunction, chronic renal failure, etc.). Multiple isoforms of sGC exist in humans, including sGC forms with α1, α2, β1 and β2 (Mayer and Koesling, 2001). sGC subunits α1/α2 share 46% sequence homology, while β1/β2 subunits share 41% sequence homology. Isoform α1β1 is the most studied, while the role of other isoforms is poorly understood, although the α2β1 complex is located in neural cells. NO receptors are found not only in mammalian cells but also in processes of bacteria across many phyla such as Cyanobacteria, Proteobacteria, Thermotogae, Firmicutes and Bacteroidetes (Boon and Marletta, 2005; Guo et al., 2018; Karow et al., 2004). The correlation between the NO signaling in humans and bacterial NO sensing was first discussed by the (Nioche et al., 2004), and (Pellicena et al., 2004) research groups, providing information about their homology ~ 15–40% in comparison to the human H-NOX domain. The detection-binding of NO by the homologous bacterial H-NOX domains plays a key role in the regulation of bacterial metabolism and in the formation of biomembrane (Plate and Marletta, 2012).
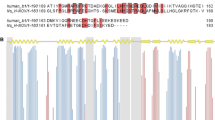
The available structural studies by X-ray crystallography do not indicate significant changes between the various complexes of bacterial H-NOX domains, either in complexes with or without diatomic gases (practically all available models show significant similarity with RMSD < 0.3Å (Makrynitsa et al., 2019). Thus, NMR conformational and dynamic studies of other bacterial H-NOX domains are gaining interest indicating sites of interaction and other conformational modifications shedding light to analogous mechanisms of action in the human H-NOX domain (Makrynitsa et al.2022, 2021; Argyriou et al., 2021; Erbil et al., 2009). Lately, structural studies have been reported regarding the NMR conformational data of Nostoc sp. H-NOX domain, as well as interaction studies of this bacterial H-NOX domain with NO and known stimulators of human sGC H-NOX domain. Npun shares 56.10% sequence homology with Nostoc sp. H-NOX domain and each one shares 39.89% (Fig. 1) and 33.86% with the human H-NOX domain respectively. Hence, Npun H-NOX is offered as a fine descriptive protein model for NMR conformational examination, analysis, and comparison with the so far reported studies.
Sequence alignment of the Human sGC β1 H-NOX domain and Nostoc punctiforme H-NOX domain. Amino acid numbering is according to the β1 sequence of human H-NOX protein domain. Color coding was selected to highlight the conserved residues with dark blue, the conserved type of residues with consensus identity > 30% with light blue and the non-conserved residues with white color
The present study reports the first backbone and side-chain assignment of the Npun H-NOX domain (1–183 residues) and the secondary elements’ prediction based on the assigned NMR chemical shifts in solution. This work will help to understand better the recognition mechanism of bacterial H-NOX domains’ discrimination and selectivity towards diatomic gases. This in turn, will unravel the role of the different and conservative amino acids in the identification process and binding of diatomic gases. Exploration of the structural and conformational changes of the H-NOX domains and the way which they differentiate among the various organisms (facultative versus obligate aerobes) may shed light on their biological role.
Methods and experiments
Protein sample preparation
H-NOX domain from Nostoc punctiforme was cloned and expressed using the pET-22b ( +) expression vector. The H-NOX protein domain comprising the residues 1–183 of the Npun H-NOX domain expressed in Escherichia coli BL21 (DE3) Star cells. The bacteria were grown in minimal medium (M9) containing 15NH4Cl (1 g/L) and 13C-glucose (4 g/L) for isotopic labelling, 0.5 mM aminolevulinic acid (δ-ALA) for enhancement of heme molecule production and ampicillin (1 mg/L) for bacterial selection. The culture was incubated in 37 °C (220 rpm) until the OD600 was approximately 0.8, after 0.5 mM IPTG induction culture incubated overnight at 18 °C (180 rpm). The protein purification procedure performed using an ion exchange column (GE Healthcare) using a NaCl gradient buffer solution. Finally, size exclusion chromatography performed using a Superdex 200 10/300GL column on an AKTA purifier 10 FPLC system (GE Healthcare). Prior to NMR analysis a 10% of 99.9% D2O was added to protein samples resulting in a concentration of 0.6 mM. UV–Vis absorption spectrum has a Soret band at 428 nm which is indicative of a Fe(II) five-coordinate heme complex (Fig. 2). These data, along with the other reports on bacterial H-NOX, strongly suggest that the Npun H-NOX protein domain is in the Fe(II) – H-NOX diamagnetic complex form (Boon et al., 2006; Tsai et al., 2012; Dai et al., 2012; Alexandropoulos et al., 2016; Makrynitsa et al., 2022).
NMR spectroscopy
The selected solvent system for all NMR experiments is the 90% H2O − 10% D2O. NMR experiments acquired at 298 K on a Bruker Avance III High Definition four-channel 700 MHz NMR spectrometer, equipped with a cryogenically cooled 5 mm 1H/13C/15 N/D Z-gradient probe (TCI). The NMR experiments for the assignment of the selected protein-domain sequence were collected as follows and followed the standard methodology. Backbone assignments for Npun H-NOX domain was obtained from the analysis of the following heteronuclear two-dimensional (2D) and three-dimensional (3D): 2D [1H-15 N] HSQC and 2D [1H-13C] HSQC, 3D HNCO, 3D HN(CA)CO, 3D TROSY-HN(CO)CACB, 3D TROSY-HNCACB, 3D HBHA(CO)NH, aliphatic 3D (H)CCH-TOCSY, 3D HNHA, 3D HNCA, 3D 15 N-NOESY, 3D 13C-NOESY aliphatic and aromatic (Davis et al., 1992, Zhang et al., 1997 and Bax and Grzesiek 1993). Additionally, a set of 3D CBCA(CO)NH modified NMR experiments were recorded to select the sequential neighbours of residues without aliphatic 13Cγ atom such as Ala, Gly, Ser, Asp, Asn, Cys and the aromatic residues, or amino acids lacking a γCO group (Ala, Ser, Cys and aromatic residues). The acquired NMR data were processed using the TopSpin 3.5 pl7 software and analysed with CARA 1.9.1.7. (Keller 2004).
Extent of assignment and data deposition
The 2D 1H-15 N HSQC NMR spectrum of Npun H-NOX shows amide signals with good dispersion, indicating a properly folded tertiary structure of the protein domain in solution. In Fig. 3 are depicted all the dispersed NH signals in the 2D 1H-15 N HSQC NMR spectrum. Analysis of the NMR spectral set resulted in the 85% assignment of 1H/15 N backbone pairs and 89.4, 87.7, 65.4, 89.5 and 89% of all Hα, Hβ, CO, Cα and Cβ chemical shifts of the Npun H-NOX. However, no backbone amide signals were detected for M1, Y2, G3, L4, L100, D101, N102, L103, H104, V107, F111, S118, L129, H160, N183. The eight Proline residues also are absent from the 2D 1H-15 N HSQC since they don’t bear the characteristic amide proton. However, assignment of the side chain for five (P43, P62, P94, P112 and P142) from the eight prolines was conducted only through the direct 13C detection experiments from the 2D 1H-13C HSQC and 1H-1H TOCSY NMR spectra. The chemical shift values for each of the assigned atoms have been deposited in the Biological Magnetic Resonance Bank (https://bmrb.io/) under the accession no. 51495. These assignment percentages are reasonably comparable to those of Ns H-NOX in its native (heme-bound) state of (Alexandropoulos et al., 2016), further corroborating their high sequential homology. Additionally, based on Cα, CO and side chain resonances the 15 amino acids V5, I46, V74, L86, L97, A105, G108, E120, E125, Y133, R134, E138, L147, I161 and Q163 were detected and assigned. In total, 30 amino acids out of 183 could not be assigned unambiguously and the vast majority are located in or form the heme pocket. As a consequence, the high flexibility of the heme and the Fe(II)-H-NOX coordination state (5-coordination complex), which is also noted to other related bacterial H-NOX domains, may have an effect on peak broadening (beyond detection) due to conformational exchange among the multiple poses adopted by the 5-coordinated heme into its cavity. All the above corroborate with the phenomenon of the large number of missing peaks (Erbil et al., 2009; Alexandropoulos et al., 2016; Makrynitsa et al., 2021; Chen et al., 2021).
Amino acids L100-H104 and G108 could not be identified and the V74, L86, L97, A105 and V107 residues lack of HN and N assignments. These amino acids are considered part of the two α-helices which are placed above and beneath the heme prosthetic group. These H-NOX sequence fragments have been already discussed and experimentally confirmed from the NMR and the crystal structures of Shewanella oneidensis (PDB: 2KIL, 2KII, 4U99) and Caldanaerobacter subterraneus H-NOX domains (PDB: 1U55) (Herzik et al., 2014; Erbil et al., 2009; Pellicena et al., 2004). In detail, the residues L86 and L97 in So H-NOX structure appears to be placed in positions where they are the last residues forming an α-helix and turning to a random coil structure. Additionally, the amino acid V74 seems to be part of the α-helix beneath the heme but also the one residue approaching closest to the heme. These three amino acids, V74, L86 and L97, seem to contribute to the same secondary elements in Npun H-NOX as well.
Identification of the proline residues cis/trans conformation was based on the analysis of the 3D TROSY HNCACB and the aliphatic 3D (H)CCH-TOCSY. Examination of the chemical shift difference of the 13Cβ and 13Cγ atoms of the identified proline residues was conducted based on the Δβγ = δ[13Cβ]- δ[13Cγ] equation (Schubert et al., 2002). The comparison resulted to a trans conformation of the five identified prolines of the Npun H-NOX domain.
Secondary structure prediction of the Npun H-NOX domain was performed using the chemical shift assignments of the atoms HN, N, Hα, CO, Cα and Cβ, for each amino acid in the sequence using TALOS + server (Shen et al., 2009). TALOS + prediction results indicate that secondary structure elements are composed from 7 α-helices and 4 β-strands organized in a αaβααααβαββ topology (Fig. 4). However, in TALOS + prediction there are two residues, 35 and 36, indicating the existence of an extended strand (E). The unassigned sequence part which comprises the residues L100-G108 is forming more likely an α-helix as it is indicated from the TALOS + prediction results for the two residues beta strand around the residues 112, as it is also reported in the X-ray structure where in Npun H-NOX domain is the area with the unassigned residues similar to Nostoc sp. H-NOX domain (Alexandropoulos et al., 2016),(Makrynitsa et al., 2021). Whereas the unassigned, annotated in grey bars in Fig. 4, residues116-118 seem to form a loop or the 118 to be the initial residue of a β-strand, as depicted in the related NMR structures of So H-NOX (PDB: 2KII, 2KIL), Sw H-NOX (PDB: 6OCV) and human b1 H-NOX (PDB: 5MNW). Summarizing, the present work describes the NMR study in solution of the Nostoc punctiforme H-NOX domain, which shares 38% sequence identity with the human sGC H-NOX domain. This analysis is a result of 3D triple resonance NMR experiments on the Npun H-NOX protein samples after following established molecular biology protocols. Signals’ dispersion of the 1H-15 N HSQC spectrum indicates a well-folded protein domain, with the almost complete sequence-specific assignment of the protein resonances revealing a mixed α/β secondary structure elements similar to Nostoc sp. H-NOX domain. The high similarity of the structural elements forming the heme cavity across many studied bacterial H-NOX domains is of great biological significance since they might act synergistically defining the ligands selectivity according to their organism functionality and biological role. Hence, the present system is of additive value to the so far similar NMR structural studies and can be exploited for comparative analysis regarding the redox switching mechanism of heme, the coordination properties of the heme iron along with the dynamics of the H-NOX domain under ligand-binding or gas sensing conditions.
Secondary structure of the heme bound Npun H-NOX as predicted by TALOS + from the chemical shift values. Color coding indicate orange for β-sheets and blue for α-helix, while grey bars point out the unassigned sequence part. Cartoon illustrations of the secondary structure elements from the Ns H-NOX X-ray structure (PDB: 2O09) and the Npun H-NOX as resulted from TALOS + prediction, are placed on top. The secondary structure elements of the Ns H-NOX X-ray structure obtained using the pdbsum (Laskowski et al., 1997)
Data availability
Assignment deposited in BMRB with ID: 51495.
References
Alexandropoulos II, Argyriou AI, Marousis KD, Topouzis S, Papapetropoulos A, Spyroulias GA (2016) 1H, 13C, 15N backbone and side-chain resonance assignment of Nostoc sp. C139A variant of the heme–nitric oxide/oxygen binding (H-NOX) domain. Biomol NMR Assign 10:395–400. https://doi.org/10.1007/s12104-016-9707-6
Argyriou AI, Makrynitsa GI, Dalkas G, Georgopoulou DA, Salagiannis K, Vazoura V, Papapetropoulos A, Topouzis S, Spyroulias GA (2021) Replacement of heme by soluble guanylate cyclase (sGC) activators abolishes heme-nitric oxide/oxygen (H-NOX) domain structural plasticity. Curr Res Struct Biol 3:324–336. https://doi.org/10.1016/j.crstbi.2021.11.003
Bax AD, Grzesiek S (1993) Methodological advances in protein NMR. Acc Chem Res 26:131–138. https://doi.org/10.1021/ar00028a001B
Boon EM, Marletta MA (2005) Ligand specificity of H-NOX domains: from sGC to bacterial NO sensors. J Inorg Biochem 99:892–902. https://doi.org/10.1016/j.jinorgbio.2004.12.016
Boon EM, Davis JH, Tran R, Karow DS, Huang SH, Pan D, Miazgowicz MM, Mathies RA, Marletta MA (2006) Nitric oxide binding to prokaryotic homologs of the soluble guanylate cyclase β1 H-NOX domain. J Biol Chem 281:21892–21902. https://doi.org/10.1074/jbc.M600557200
Chen CY, Lee W, Renhowe PA, Jung J, Montfort WR (2021) Solution structures of the Shewanella woodyi H-NOX protein in the presence and absence of soluble guanylyl cyclase stimulator IWP-051. Protein Sci 30:448–463. https://doi.org/10.1002/pro.4005
Dai Z, Farquhar ER, Arora DP, Boon EM (2012) Is histidine dissociation a critical component of the NO/H-NOX signaling mechanism? Insights from X-ray absorption spectroscopy. Dalton Trans 41:7984–7993. https://doi.org/10.1039/C2DT30147D
Davis AL, Keeler J, Laue ED, Moskau D (1992) Experiments for recording pure-absorption heteronuclear correlation spectra using pulsed field gradients. J Magn Reson (1969) 98:207–216. https://doi.org/10.1016/0022-2364(92)90126-R
Erbil WK, Price MS, Wemmer DE, Marletta MA (2009) A structural basis for H-NOX signaling in Shewanella oneidensis by trapping a histidine kinase inhibitory conformation. Proc Natl Acad Sci 106:19753–19760. https://doi.org/10.1073/pnas.0911645106
Guo Y, Cooper MM, Bromberg R, Marletta MA (2018) A dual-H-NOX Signaling system in Saccharophagus degradans. Biochemistry 57:6570–6580. https://doi.org/10.1021/acs.biochem.8b01058
Herzik MA, Jonnalagadda R, Kuriyan J, Marletta MA (2014) Structural insights into the role of iron–histidine bond cleavage in nitric oxide-induced activation of H-NOX gas sensor proteins. Proc Natl Acad Sci 111:E4156–E4164. https://doi.org/10.1073/pnas.1416936111
Karow DS, Pan D, Tran R, Pellicena P, Presley A, Mathies RA, Marletta MA (2004) Spectroscopic characterization of the soluble guanylate cyclase-like heme domains from Vibrio cholerae and Thermoanaerobacter tengcongensis. Biochemistry 43:10203–10211. https://doi.org/10.1021/bi049374l
Keller R (2004) The computer aided resonance assignment tutorial CH-6410. Cantina Verlag, Goldau
Laskowski RA, Hutchinson EG, Michie AD, Wallace AC, Jones ML, Thornton JM (1997) PDBsum: a Web-based database of summaries and analyses of all PDB structures. Trends Biochem Sci 22:488–490. https://doi.org/10.1016/S0968-0004(97)01140-7
Makrynitsa GI, Zompra AA, Argyriou AI, Spyroulias GA, Topouzis S (2019) Therapeutic targeting of the soluble guanylate cyclase. Curr Med Chem 26:2730–2747. https://doi.org/10.2174/0929867326666190108095851
Makrynitsa GI, Argyriou AI, Dalkas G, Georgopoulou DA, Bantzi M, Giannis A, Papapetropoulos A, Spyroulias GA (2021) Backbone and side chain NMR assignments of the H-NOX domain from Nostoc sp. in complex with BAY58-2667 (cinaciguat). Biomol NMR Assign 15:53–57. https://doi.org/10.1007/s12104-020-09991-2
Makrynitsa G, Argyriou AI, Zompra AA, Salagiannis K, Vazoura V, Papapetropoulos A, Topouzis S, Spyroulias GA (2022) Mapping of the sGC stimulator BAY 41–2272 binding site on H-NOX domain and its regulation by the redox state of the heme. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2022.925457
Mayer B, Koesling D (2001) cGMP signalling beyond nitric oxide. Trends Pharmacol Sci 22:546–548. https://doi.org/10.1016/S0165-6147(00)01889-7
Nioche P, Berka V, Vipond J, Minton N, Tsai A-L, Raman C (2004) Femtomolar sensitivity of a NO sensor from Clostridium botulinum. Science 306:1550–1553. https://doi.org/10.1126/science.1103596
Papapetropoulos A, Hobbs AJ, Topouzis S (2015) Extending the translational potential of targeting NO/cGMP-regulated pathways in the CVS. Br J Pharmacol 172:1397–1414. https://doi.org/10.1111/bph.12980
Pellicena P, Karow DS, Boon EM, Marletta MA, Kuriyan J (2004) Crystal structure of an oxygen-binding heme domain related to soluble guanylate cyclases. Proc Natl Acad Sci 101:12854–12859. https://doi.org/10.1073/pnas.0405188101
Plate L, Marletta MA (2012) Nitric oxide modulates bacterial biofilm formation through a multicomponent cyclic-di-GMP signaling network. Mol Cell 46:449–460. https://doi.org/10.1016/j.molcel.2012.03.023
Schubert M, Labudde D, Oschkinat H, Schmieder P (2002) A software tool for the prediction of Xaa-Pro peptide bond conformations in proteins based on 13C chemical shift statistics. J Biomol NMR 24:149–154. https://doi.org/10.1023/A:1020997118364
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44:213–223. https://doi.org/10.1007/s10858-009-9333-z
Stone JR, Marletta MA (1996) Spectral and kinetic studies on the activation of soluble guanylate cyclase by nitric oxide. Biochemistry 35:1093–1099. https://doi.org/10.1021/bi9519718
Tsai AL, Martin E, Berka V, Olson JS (2012) How do heme-protein sensors exclude oxygen? Lessons learned from cytochrome c′, Nostoc puntiforme heme nitric oxide/oxygen-binding domain, and soluble guanylyl cyclase. ARS 17:1246–1263. https://doi.org/10.1089/ars.2012.4564
Zhang O, Forman-Kay JD, Shortle D, Kay LE (1997) Triple-resonance NOESY-based experiments with improved spectral resolution: applications to structural characterization of unfolded, partially folded and folded proteins. J Biomol NMR 9:181–200
Acknowledgements
This work was supported by the INSPIRED (MIS 5002550) which is implemented under the Action ‘Reinforcement of the Research and Innovation Infrastructure,’ funded by the Operational Program ‘Competitiveness, Entrepreneurship and Innovation’ (NSRF 2014–2020) and co-financed by Greece and the European Union (European Regional Development Fund). EU FP7 REGPOT CT-2011-285950—“SEE-DRUG” project is acknowledged for the purchase of UPAT’s 700 MHz NMR equipment. SC wish to acknowledge co-financed by Greece and the European Union (European Social Fund) through the Operational Programme "Human Resources Development, Education and Lifelong Learning" in the context of the Action "Enhancing Human Resources Research Potential by undertaking a Doctoral Research" Sub-Action 2: IKY Scholarship Programme for PhD Candidates in the Greek Universities (MIS 5113934).
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
Styliani A. Chasapi and Aikaterini I. Argyriou have equally contributed to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chasapi, S.A., Argyriou, A.I. & Spyroulias, G.A. Backbone and side chain NMR assignment of the heme-nitric oxide/oxygen binding (H-NOX) domain from Nostoc punctiforme. Biomol NMR Assign 16, 379–384 (2022). https://doi.org/10.1007/s12104-022-10107-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-022-10107-1