Abstract
PRK1 is a member of the protein kinase C-related kinase (PRK) family of serine/threonine kinases and a downstream effector of Rho GTPases. PRK1 has three N-terminal Homology Region 1 (HR1) domains (HR1a, HR1b and HR1c), which form antiparallel coiled coils that interact with Rho family GTPases. PRK1 also has a C2-like domain that targets it to the plasma membrane and a kinase domain, which is a member of the protein kinase C superfamily. PRK1 is involved in cytoskeletal regulation, cell adhesion, cell cycle progression and the immune response, and is implicated in cancer. There is currently no structural information for the HR1c domain. The 1H, 15N and 13C NMR backbone and sidechain resonance assignment of the HR1c domain presented here forms the basis for this domain’s structural characterisation. This work will also enable studies of interactions between the three HR1 domains in an effort to obtain structural insight into the regulation of PRK1 activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Biological context
Protein kinase C-related kinase 1 (PRK1) is a member of the PRK family of serine/threonine kinases, which is within the AGC group of kinases. The PRK proteins were first identified as effector kinases of the small G protein RhoA (Watanabe et al. 1996; Amano et al. 1996). Several roles of PRK1 in cytoskeletal regulation have been described, including phosphorylation of vimentin (Matsuzawa et al. 1997), neurofilament assembly disruption (Manser et al. 2008), interaction with the actin cross-linking protein α-actinin (Mukai et al. 1997) and insulin-induced stress fibre disruption (Dong et al. 2000). PRK1 has also been linked to androgen receptor signalling and transcriptional regulation (Metzger et al. 2003, 2008), the immune response (Park et al. 2016), cell cycle progression (Schmidt et al. 2007), thromboxane signalling (O’Sullivan et al. 2015) and mTOR signalling (Yang et al. 2017; Wallroth et al. 2019). PRK1 is overexpressed in human prostate cancer (Metzger et al. 2003; O’Sullivan et al. 2017) and has also been implicated in ovarian cancer (Galgano et al. 2009) and in the migration of bladder tumour cells (Lachmann et al. 2011).
PRK1 has three N-terminal HR1 domains, HR1a, HR1b and HR1c. These are antiparallel coiled coils of approximately 10 kDa that interact with the nucleotide-sensitive switch regions of Rho GTPases as shown in the structures of RhoA with HR1a (Maesaki et al. 1999) and Rac1 with HR1b (Modha et al. 2008). PRK1 also has a C2-like domain, which targets the protein to the plasma membrane (reviewed in Corbalán-García and Gómez-Fernández 2010) and a C-terminal catalytic domain, which belongs to the protein kinase C family (reviewed in Pearce et al. 2010; Arencibia et al. 2013; Chamberlain et al. 2014). The three HR1 domains together with the C2 domain comprise the regulatory region of the PRKs.
PRK1 is activated by phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylation of its activation loop (Dong et al. 2000) and by lipids such as PIP2, PIP3 and arachidonic acid (Palmer et al. 1995; Mukai et al. 1994; Morrice et al. 1994; Kitagawa et al. 1995; Peng et al. 1996; Yu et al. 1997; Mukai and Ono 2006; Falk et al. 2014). Small GTPases like RhoA and Rac1 can also activate PRK1 activity (Amano et al. 1996; Lu and Settleman 1999) and it is thought that RhoA binds to HR1a to relieve autoinhibition mediated by a pseudosubstrate region in this domain (Kitagawa et al. 1996).
The HR1a and HR1b domains both bind to Rho proteins and their structures have been characterised. There is currently no structural information on the HR1c domain and it is unknown whether this domain is structured and what role it plays in PRK1 regulation. There is also no information on the entire HR1 region and particularly on whether the three HR1 domains interact with each other or exist independently in solution. Here we present the 1H, 15N and 13C NMR resonance assignment of the PRK1 HR1c domain. This will form the basis for structure determination and for investigating interactions between the three PRK1 HR1 domains, providing much needed structural insight into PRK1 regulation.
Methods and experiments
Protein expression and purification
Human PRK1 HR1c (residues 201–297) was expressed in pGEXHISP (Hutchinson et al. 2011) with a stop codon engineered to prevent the translation of the C-terminal His6 tag. The HR1c domain was expressed as a GST fusion in E.coli BL21 (DE3) Rosetta2 pLysS. Overnight cultures were diluted 1 in 10, grown at 37 oC to an A600 of 0.8 and induced by adding 0.1 mM isopropyl β-d-1-thiogalactopyranoside. The cultures were then incubated at 20 oC for 20 h. For NMR experiments, isotopically labelled protein was produced by growing E.coli in M9 media supplemented with 1 g/L 15NH4Cl (Sigma-Aldrich) and 3 g/L 13C-glucose (Cambridge Isotope Laboratories). The protein was purified using glutathione agarose beads, eluted by adding HRV 3C protease to cleave the N-terminal GST-tag, further purified by size exclusion chromatography on a Superdex75 16/60 column (GE Healthcare) and concentrated in an Amicon Ultra-4 Centrifugal Filter Unit (Millipore). Protein concentration was determined by amino acid analysis (Protein and Nucleic Acid Chemistry Facility, Department of Biochemistry, University of Cambridge).
NMR spectroscopy
NMR spectra were recorded at 298 K with 1.6 mM 15N-labelled HR1c or 1.2 mM 15N, 13C-labelled HR1c in 20 mM sodium phosphate pH 7.3, 150 mM NaCl, 0.05% NaN3, 10% D2O. 15N-HSQC, 15N-separated NOESY (150 ms mixing time), 15N-separated TOCSY (60 ms mixing time), HNCA, HNCACB, HN(CO)CA, HN(CO)CACB, 13C-HSQC and 13C-separated HCCH-TOCSY experiments were recorded on a Bruker DRX500. A 13C-separated NOESY experiment (100 ms mixing time) was recorded on a Bruker AV800. NMR data were processed using Azara (Wayne Boucher, University of Cambridge).
Backbone and side-chain resonance assignment was carried out in CCPN Analysis v2.3 (Vranken et al. 2005). Standard methodology (Gardner and Kay 1998) was used to carry out the backbone assignment using the 15N-separated NOESY, 15N-separated TOCSY, HNCA, HNCACB, HN(CO)CA, HN(CO)CACB experiments with reference to the 15N-HSQC experiment. The 13C-separated HCCH-TOCSY and 13C-separated NOESY experiments were used with reference to the 13C-HSQC, in addition to the above experiments, for the assignment of side-chain resonances. Side-chain amides were assigned using the 15N-separated NOESY and the 13C-separated NOESY. Aromatic side-chains were assigned using the 15N-separated NOESY and the 13C-separated NOESY spectra with reference to the aromatic region of the 13C-HSQC spectrum.
Extent of assignment and data deposition
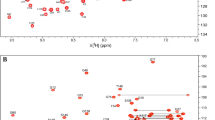
The PRK1 HR1c domain gave well-dispersed spectra as shown by the quality of the 15N HSQC spectrum (δΗ = 6.4–10.1 ppm and δN = 104–126 ppm) (Fig. 1). The construct used includes PRK1 HR1c residues 201–297 and an additional 7 N-terminal residues (GPLGSHM) encoded by the expression vector, and the majority of resonances were assigned (Table 1). All the backbone NH resonances were assigned, with the exception of the His in the N-terminal linker. All Asn and Gln NH2 resonances were assigned. Nine out of the 10 expected Arg HεNε peaks were observed in the HSQC (Fig. 1) but could not be unambiguously assigned, although one Arg Hε was assigned in the 13C-separated NOESY. All other sidechain resonances were assigned except those that rapidly exchange with the solvent: OH groups, primary amines (N-terminal and Lys sidechains), Arg Hη/Nη, and His Hδ1/Nδ1 and Hε2/Nε2.
15N-HSQC recorded on 1.6 mM 15N-labelled PRK1 HR1c in 20 mM sodium phosphate pH 7.3, 150 mM NaCl and 10% D2O on a Bruker DRX500 at 298 K. The assignments are indicated for all assigned resonances. The arginine side-chain HεNε resonances are aliased in the 15N dimension and are indicated by an asterisk
A Chemical Shift Index (CSI) of + 1 or −1 was generated from the difference in backbone chemical shifts (Cα, Cβ and Hα) from the random coil positions (Wishart and Sykes 1994). Figure 2 shows the short-range NOEs and the CSI for each residue. The backbone chemical shifts were also submitted to TALOS-N (Shen and Bax 2013) to predict the protein’s secondary structure, which is also shown in Fig. 2. Taken together the CSI, TALOS-N and short-range NOEs allow the secondary structure to be determined. The data suggest that the PRK1 HR1c domain comprises three α-helices that are connected by short, unstructured regions. The first two helices are likely to form an anti-parallel coiled coil akin to other HR1 domains whose structures are known: the PRK1 HR1a (Maesaki et al. 1999), PRK1 HR1b (Owen et al. 2003), TOCA1 HR1 (Watson et al. 2016) and CIP4 HR1 (Kobashigawa et al. 2009) domains. Chemical shift assignment of the PRK1 HR1c domain will enable the structure determination using distance and torsion angle restraints determined from NMR experiments. The full backbone assignment will also allow investigation into inter-domain interactions between the three HR1 domains in PRK1. This will provide a structural understanding of how PRK1 activity may be regulated. The chemical shifts have been deposited in the BMRB, accession number 50216.
Summary of secondary structure of the PRK1 HR1c domain. The plot summarises the sequential and medium-range NOEs observed in 15N-separated and 13C-separated NOESY experiments. The height of the bars represents the strength of the following NOEs: (1) dαN (dαδ)—NOEs between Hα of residue i and NH of residue i + 1 or Hδ of an i + 1 Proline; (2) dNN (dNδ)—NH of residue i and NH of residue i + 1 or Hδ of an i + 1 Proline. The next 2 rows show the short-range, i to i + 3 NOEs observed in α-helical regions. Δδ represents the chemical shift deviation from random coil for 13Cα, 13Cβ and 1Hα where the size of the bar represents the size of the shift deviation. The overall score for the secondary shifts, the chemical shift index (CSI), is represented as grey boxes. A value of + 1 denotes an extended, β-sheet-like structure and a value of −1 an α-helical structure. Secondary structure was predicted from the chemical shifts using TALOS-N and is shown with a cartoon representation
References
Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K (1996) Identification of a putative target for Rho as the serine-threonine kinase protein kinase N. Science 271:648–650
Arencibia JM, Pastor-Flores D, Bauer AF, Schulze JO, Biondi RM (2013) AGC protein kinases: from structural mechanism of regulation to allosteric drug development for the treatment of human diseases. Biochim Biophys Acta 1834:1302–1321
Chamberlain P, Delker S, Pagarigan B, Mahmoudi A, Jackson P, Abbasian M, Muir J, Raheja N, Cathers B (2014) Crystal structures of PRK1 in complex with the clinical compounds lestaurtinib and tofacitinib reveal ligand induced conformational changes. PLoS ONE 9:e103638
Corbalán-García S, Gómez-Fernández JC (2010) The C2 domains of classical and novel PKCs as versatile decoders of membrane signals. BioFactors 36:1–7
Dong LQ, Landa LR, Wick MJ, Zhu L, Mukai H, Ono Y, Liu F (2000) Phosphorylation of protein kinase N by phosphoinositide-dependent protein kinase-1 mediates insulin signals to the actin cytoskeleton. Proc Natl Acad Sci USA 97:5089–5094
Falk MD, Liu W, Bolanos B, Unsal-Kacmaz K, Klippel A, Grant S, Brooun A, Timofeevski S (2014) Enzyme kinetics and distinct modulation of the protein kinase N family of kinases by lipid activators and small molecule inhibitors. Biosci Rep 34:93–106
Galgano MT, Conaway M, Spencer AM, Paschal BM, Frierson HF Jr (2009) PRK1 distribution in normal tissues and carcinomas: overexpression and activation in ovarian serous carcinoma. Hum Pathol 40:1434–1440
Gardner KH, Kay LE (1998) The use of 2H, 13C, 15N multidimensional NMR to study the structure and dynamics of proteins. Annu Rev Biophys Biomol Struct 27:357–406
Hutchinson CL, Lowe PN, McLaughlin SH, Mott HR, Owen D (2011) Mutational analysis reveals a single binding interface between RhoA and its effector, PRK1. Biochemistry 50:2860–2869
Kitagawa M, Mukai H, Shibata H, Ono Y (1995) Purification and characterization of a fatty acid-activated protein kinase (PKN) from rat testis. Biochem J 310:657–664
Kitagawa M, Shibata H, Toshimori M, Mukai H, Ono Y (1996) The role of the unique motifs in the amino-terminal region of PKN on its enzymatic activity. Biochem Biophys Res Commun 220:963–968
Kobashigawa Y, Kumeta H, Kanoh D, Inagaki F (2009) The NMR structure of the TC10- and Cdc42-interacting domain of CIP4. J Biomol NMR 44:113–118
Lachmann S, Jevons A, De Rycker M, Casamassima A, Radtke S, Collazos A, Parker PJ (2011) Regulatory domain selectivity in the cell-type specific PKN-dependence of cell migration. PLoS ONE 6:e21732
Lu Y, Settleman J (1999) The Drosophila Pkn protein kinase is a Rho/Rac effector target required for dorsal closure during embryogenesis. Genes Dev 13:1168–1180
Maesaki R, Ihara K, Shimizu T, Kuroda S, Kaibuchi K, Hakoshima T (1999) The structural basis of Rho effector recognition revealed by the crystal structure of human RhoA complexed with the effector domain of PKN/PRK1. Mol Cell 4:793–803
Manser C, Stevenson A, Banner S, Davies J, Tudor EL, Ono Y, Leigh PN, McLoughlin DM, Shaw CE, Miller CCJ (2008) Deregulation of PKN1 activity disrupts neurofilament organisation and axonal transport. FEBS Lett 582:2303–2308
Matsuzawa K, Kosako H, Inagaki N, Shibata H, Mukai H, Ono Y, Amano M, Kaibuchi K, Matsuura Y, Azuma I, Inagaki M (1997) Domain-specific phosphorylation of vimentin and glial fibrillary acidic protein by PKN. Biochem Biophys Res Commun 234:621–625
Metzger E, Müller JM, Ferrari S, Buettner R, Schüle R (2003) A novel inducible transactivation domain in the androgen receptor: implications for PRK in prostate cancer. EMBO J 22:270–280
Metzger E, Yin N, Wissmann M, Kunowska N, Fischer K, Friedrichs N, Patnaik D, Higgins JMG, Potier N, Scheidtmann KH, Buettner R, Schüle R (2008) Phosphorylation of histone H3 at threonine 11 establishes a novel chromatin mark for transcriptional regulation. Nat Cell Biol 10:53–60
Modha R, Campbell LJ, Nietlispach D, Buhecha HR, Owen D, Mott HR (2008) The Rac1 polybasic region is required for interaction with its effector PRK1. J Biol Chem 283:1492–1500
Morrice NA, Gabrielli B, Kemp BE, Wettenhall REH (1994) A cardiolipin-activated protein kinase from rat liver structurally distinct from the protein kinases C. J Biol Chem 269:20040–20046
Mukai H, Ono Y (2006) Purification and kinase assay of PKN. Methods Enzymol 406:234–250
Mukai H, Kitagawa M, Shibata H, Takanaga H, Mori K, Shimakawa M, Miyahara M, Hirao K, Ono Y (1994) Activation of PKN, a novel 120-kDa protein kinase with leucine zipper-like sequences, by unsaturated fatty acids and by limited proteolysis. Biochem Biophys Res Commun 204:348–356
Mukai H, Toshimori M, Shibata H, Takanaga H, Kitagawa M, Miyahara M, Shimakawa M, Ono Y (1997) Interaction of PKN with α-actinin. J Biol Chem 272:4740–4746
O’Sullivan AG, Mulvaney EP, Hyland PB, Kinsella BT (2015) Protein kinase C-related kinase 1 and 2 play an essential role in thromboxane-mediated neoplastic responses in prostate cancer. Oncotarget 6:26437–26456
O’Sullivan AG, Mulvaney EP, Kinsella BT (2017) Regulation of protein kinase C-related kinase (PRK) signalling by the TPα and TPβ isoforms of the human thromboxane A2 receptor: implications for thromboxane- and androgen- dependent neoplastic and epigenetic responses in prostate cancer. Biochim Biophys Acta 1863:838–856
Owen D, Lowe PN, Nietlispach D, Brosnan CE, Chirgadze DY, Parker PJ, Blundell TL, Mott HR (2003) Molecular dissection of the interaction between the small G proteins Rac1 and RhoA and protein kinase C-related kinase 1 (PRK1). J Biol Chem 278:50578–50587
Palmer RH, Dekker LV, Woscholski R, Le Good JA, Gigg R, Parker PJ (1995) Activation of PRK1 by phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 270:22412–22416
Park YH, Wood G, Kastner DL, Chae JJ (2016) Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol 17:914–921
Pearce LR, Komander D, Alessi DR (2010) The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11:9–22
Peng B, Morrice NA, Groenen LC, Wettenhall REH (1996) Phosphorylation events associated with different states of activation of a hepatic cardiolipin/protease-activated protein kinase. J Biol Chem 271:32233–32240
Schmidt A, Durgan J, Magalhaes A, Hall A (2007) Rho GTPases regulate PRK2/PKN2 to control entry into mitosis and exit from cytokinesis. EMBO J 26:1624–1636
Shen Y, Bax A (2013) Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J Biomol NMR 56:227–241
Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59:687–696
Wallroth A, Koch PA, Marat AL, Krause E, Haucke V (2019) Protein kinase N controls a lysosomal lipid switch to facilitate nutrient signalling via mTORC1. Nat Cell Biol 21:1093–1101
Watanabe G, Saito Y, Madaule P, Ishizaki T, Fujisawa K, Morii N, Mukai H, Ono Y, Kakizuka A, Narumiya S (1996) Protein kinase N (PKN) and PKN-related protein rhophilin as targets of small GTPase Rho. Science 271:645–648
Watson JR, Fox HM, Nietlispach D, Gallop JL, Owen D, Mott HR (2016) Investigation of the interaction between Cdc42 and its effector TOCA1. J Biol Chem 291:13875–13890
Wishart DS, Sykes BD (1994) The 13C Chemical-Shift Index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR 4:171–180
Yang CS, Melhuish TA, Spencer A, Ni L, Hao Y, Jividen K, Harris TE, Snow C, Frierson HF Jr, Wotton D, Paschal BM (2017) The protein kinase C super-family member PKN is regulated by mTOR and influences differentiation during prostate cancer progression. Prostate 77:1452–1467
Yu W, Liu J, Morrice NA, Wettenhall REH (1997) Isolation and characterization of a structural homologue of human PRK2 from rat liver: distinguishing substrate and lipid activator specificities. J Biol Chem 272:10030–10034
Funding
This work was supported by the A.G. Leventis Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sophocleous, G., Wood, G., Owen, D. et al. 1H, 15N and 13C resonance assignments of the HR1c domain of PRK1, a protein kinase C-related kinase. Biomol NMR Assign 14, 245–250 (2020). https://doi.org/10.1007/s12104-020-09954-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-020-09954-7