Abstract
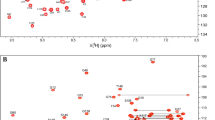
K-Ras exists in two distinct structural conformations specific to binding of GDP and GTP nucleotides. The cycling between an inactive, GDP-bound state and an active, GTP-bound state is regulated by guanine nucleotide exchange factors and GTPase activating proteins, respectively. The activated form of K-Ras regulates cell proliferation, differentiation and survival by controlling several downstream signaling pathways. Oncogenic mutations that attenuate the GTPase activity of K-Ras result in accumulation of this key signaling protein in its hyperactivated state, leading to uncontrolled cellular proliferation and tumorogenesis. Mutations at position 12 are the most prevalent in K-Ras associated cancers, hence K-RasG12C has become a recent focus of research for therapeutic intervention. Here we report 1HN, 15N, and 13C backbone and 1H, 13C side-chain resonance assignments for the 19.3 kDa (aa 1–169) human K-Ras protein harboring an oncogenic G12C mutation in the active GppNHp-bound form (K-RasG12C-GppNHp), using heteronuclear, multidimensional NMR spectroscopy at 298K. Triple-resonance data assisted the assignments of the backbone 1H, 15N, and 13C resonances of 126 out of 165 non-proline residues. The vast majority of unassigned residues are exchange-broadened beyond detection on the NMR time scale and belong to the P-loop and two flexible Switch regions.


Similar content being viewed by others
References
Boriack-Sjodin PA, Margarit SM, Bar-Sagi D, Kuriyan J (1998) The structural basis of the activation of Ras by Sos. Nature 394(6691):337–343. https://doi.org/10.1038/28548
Buhrman G, O’Connor C, Zerbe B, Kearney BM, Napoleon R, Kovrigina EA, Vajda S, Kozakov D, Kovrigin EL, Mattos C (2011) Analysis of binding site hot spots on the surface of Ras GTPase. J Mol Biol 413(4):773–789. https://doi.org/10.1016/j.jmb.2011.09.011
Castellano E, Santos E (2011) Functional specificity of ras isoforms: so similar but so different. Genes Cancer 2(3):216–231. https://doi.org/10.1177/1947601911408081
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMR pipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Downward J (2003) Targeting Ras signalling pathways in cancer therapy. Nat Rev Cancer 3(1):11–22. https://doi.org/10.1038/nrc969
Güntert P, Mumenthaler C, Wüthrich K (1997) Torsion angle dynamics for NMR structure calculation with the new program DYANA. J Mol Biol 273(1):283–298. https://doi.org/10.1006/jmbi.1997.1284
Hafsa NE, Arndt D, Wishart DS (2015) CSI 3.0: a web server for identifying secondary and super-secondary structure in proteins using NMR chemical shifts. Nucleic Acids Res 43:W370–W377. https://doi.org/10.1093/nar/gkv494
Hunter JC, Gurbani D, Ficarro SB, Carrasco MA, Lim SM, Choi HG, Xie T, Marto JA, Chen Z, Gray NS, Westover KD (2014) In situ selectivity profiling and crystal structure of SML-8-73-1, an active site inhibitor of oncogenic K-Ras G12C. Proc Natl Acad Sci USA 111:8895–8900. https://doi.org/10.1073/pnas.1404639111
Ito Y, Yamasaki K, Iwahara J, Terada T, Kamiya A, Shirouzu M, Muto Y, Kawai G, Yokoyama S, Laue ED, Wälchli M, Shibata T, Nishimura S, Miyazawa T (1997) Regional polysterism in the GTP-bound form of the human c-Ha-Ras protein. Biochemistry 36(30):9109–9119. https://doi.org/10.1021/bi970296u
Janes MR, Zhang J, Li LS, Hansen R, Peters U, Guo X, Chen Y, Babbar A, Firdaus SJ, Darjania L, Feng J, Chen JH, Li S, Li S, Long YO, Thach C, Liu Y, Zarieh A, Ely T, Kucharski JM, Kessler LV, Wu T, Yu K, Wang Y, Yao Y, Deng X, Zarrinkar PP, Brehmer D, Dhanak D, Lorenzi MV, Hu-Lowe D, Patricelli MP, Ren P, Liu Y (2018) Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell 172(3):578–589. https://doi.org/10.1016/j.cell.2018.01.006
Kauke MJ, Traxlmayr MW, Parker JA, Kiefer JD, Knihtila R, McGee J, Verdine G, Mattos C, Wittrup KD (2017) An engineered protein antagonist of K-Ras/B-Raf interaction. Sci Rep. 19;7(1):5831. https://doi.org/10.1038/s41598-017-05889-7
O’Connor C, Kovrigin EL (2012) Assignments of backbone 1H, 13C and 15N resonances in H-Ras (1–166) complexed with GppNHp at physiological pH. Biomol NMR Assign 6:91–93. https://doi.org/10.1007/s12104-011-9332-3
Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM (2013) K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503(7477):548–551. https://doi.org/10.1038/nature12796
Prior IA, Lewis PD, Mattos C (2012) A comprehensive survey of Ras mutations in cancer. Cancer Res 72:2457–2467. https://doi.org/10.1158/0008-5472.CAN-11-2612
Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D (2011) Ras oncogenes: weaving a tumorigenic web. Nat Rev Cancer 11:761–774. https://doi.org/10.1038/nrc3106
Salzmann M, Wider G, Pervushin K, Senn H, Wuthrich K (1999) TROSY-type triple-resonance experiments for sequential NMR assignments of large proteins. J Amer Chem Soc 121(4):844–848. https://doi.org/10.1021/ja9834226
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR methods for the structure determination of proteins in solution employing pulsed field gradients. Prog NMR Spectrosc 34:93–158
Sharma AK, Lee SJ, Rigby AC, Townson SA (2018) NMR 1H,13C, 15N backbone and 13C side chain resonance assignment of the G12C mutant of human K-Ras bound to GDP. Biomol NMR Assign. https://doi.org/10.1007/s12104-018-9821-8
Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59:687–696. https://doi.org/10.1002/prot.20449
Acknowledgements
All NMR experiments were performed at Brandeis University NMR facility on a Bruker spectrometer of 800 MHz equipped with cryoprobe.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, A.K., Lee, SJ., Zhou, M. et al. NMR 1H,13C, 15N resonance assignment of the G12C mutant of human K-Ras bound to GppNHp. Biomol NMR Assign 13, 227–231 (2019). https://doi.org/10.1007/s12104-019-09882-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-019-09882-1