Abstract
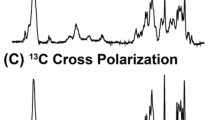
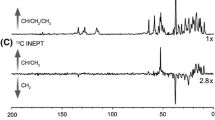
The voltage dependent anion channel (VDAC) forms a channel for metabolites and nutrients in the outer membrane of mitochondria, and it is also involved in apoptotic pathways. Here, we report sequence-specific NMR assignments for the isoform 1 of human VDAC reconstituted in lauryldimethylamine oxide (LDAO) detergent micelles. The assignments were deposited in the BMRB data base with accession number 16381.



Similar content being viewed by others
References
Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M, Zeth K (2008) Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci USA 105:15370–15375
Colombini M (1979) A candidate for the permeability pathway of the outer mitochondrial membrane. Nature 279:643–645
Colombini M (2004) VDAC: The channel at the interface between mitochondria and the cytosol. Mol Cell Biochem 256:107–115
Goto NK, Gardner KH, Mueller GA, Willis RC, Kay LE (1999) A robust and cost-effective method for the production of Val, Leu, Ile (δ1) methyl-protonated 15N-, 13C-, 2H-labeled proteins. J Biomol NMR 13:369–374
Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G (2008) Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 321:1206–1210
Hiller S, Ibraghimov I, Wagner G, Orekhov VY (2009) Coupled decomposition of four-dimensional NOESY spectra. J Am Chem Soc 131:12970–12978
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87:99–163
Malia TJ, Wagner G (2007) NMR structural investigation of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic Bcl-xL. Biochemistry 46:514–525
Pervushin K, Riek R, Wider G, Wüthrich K (1997) Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci USA 94:12366–12371
Shimizu S, Narita M, Tsujimoto Y (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399:483–487
Tugarinov V, Kay LE (2003) Ile, Leu, and Val methyl assignments of the 723-residue malate synthase G using a new labeling strategy and novel NMR methods. J Am Chem Soc 125:13868–13878
Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, Ping P, Abramson J (2008) The crystal structure of mouse VDAC1 at 2.3 Å resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA 105:17742–17747
Acknowledgments
This work was supported by the United States National Institute of Health by the roadmap grant GM075879 and the grants GM066360, GM47467 and EB 002026 for purchase, operation and maintenance of the instruments. S.H. was supported in part by the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hiller, S., Malia, T.J., Garces, R.G. et al. Backbone and ILV side chain methyl group assignments of the integral human membrane protein VDAC-1. Biomol NMR Assign 4, 29–32 (2010). https://doi.org/10.1007/s12104-009-9194-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-009-9194-0