Abstract
Objective
The primary goal of this study was to investigate the expressions of TUFT1 (Tuftelin) and Rac1-GTP in the cancerous tissues of individuals with triple-negative breast cancer (TNBC). Additionally, we aimed to explore the correlation between TUFT1 and Rac1-GTP expressions and examine the associations of TUFT1 and Rac1-GTP expressions with the clinical and pathological indicators of the patients.
Methods
Ninety-six patients diagnosed with TNBC, scheduled for surgery between May 2022 and November 2022, were enrolled in this study. Cancerous tissue specimens were collected from these patients, and immunohistochemistry was employed to evaluate the levels of TUFT1 and Rac1-GTP expressions in the cancerous tissues. Subsequent to data collection, a comprehensive analysis was conducted to examine the correlation between TUFT1 and Rac1-GTP expressions. Furthermore, we sought to assess the associations of TUFT1 and Rac1-GTP expressions with the clinical and pathological indicators of the patients.
Results
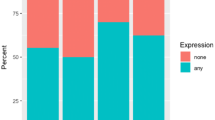
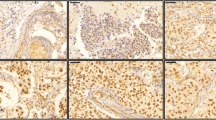
The TUFT1 protein was expressed in both the membrane and cytoplasm of TNBC cancer cells, with notably higher expression observed in the cytoplasm. Rac1-GTP was primarily expressed in the cytoplasm. There was a positive correlation between the levels of TUFT1 and Rac1-GTP expressions (χ2 = 9.816, P < 0.05). The levels of TUFT1 and Rac1-GTP protein expressions showed no correlation with patient age (χ2 = 2.590, 2.565, P > 0.05); however, they demonstrated a positive correlation with tumor size (χ2 = 5.592,5.118), histological grading (χ2 = 6.730, 5.443), and lymph node metastasis (χ2 = 8.221, 5.180) (all with a significance level of P < 0.05).
Conclusion
A significant correlation was identified between the levels of TUFT1 and Rac1-GTP expressions in the cancerous tissues of patients with TNBC, suggesting a close association with the progression of TNBC. The two molecules play significant roles in facilitating an early diagnosis and treatment of TNBC.

Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- DAB:
-
Diaminobenzidine
- GDP:
-
Guanosine diphosphate
- GTP:
-
Guanosine triphosphate
- TNBC:
-
Triple-negative breast cancer
- TUFT1:
-
Tuftelin
References
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–90.
Li Y, Shi XY, Sui X. Clinicopathological features and prognostic analysis of triple negative breast cancer. J Aerospace Med. 2021;32(2):135–7.
Gao XJ, Lv P, Zhuang H. Analysis of clinical problems in endodontic diseases. I. Clinical types and molecular biological pathogenesis of enamel dysplasia. Chin J Stomatol. 2009;2009(5):314–7.
Wu MN, Yang JL, Shen SJ, Wang L, Zheng WJ, Yao M, et al. Expression and clinical value of enamel plexin in HBV-associated hepatocellular carcinoma. Chin J Hepatol. 2021;29(4):338–43.
Wang CY, Liang BH, Wu JY, Tang ZH, Xu XW, Yin ZJ, et al. Research progress in the regulation of RAC1 on malignant tumors and its molecular mechanism. Carcinogenesis Teratogenesis Mutagenesis. 2021;33(5):401–4.
Liu WG, Du J, Zhao M, Zhang L, Lin ZN, Chen B. Clinical implications of TUFT1 protein expression and correlation with RelA protein in breast cancer. Int J Clin Exp Pathol. 2017;10(6):6544–51.
Zhao S, Ma D, Xiao Y, Jiang YZ, Shao ZM. The clinicopathological features and survival outcomes of different histological subtypes in triple-negative breast cancer. J Cancer. 2018;9(2):296–303.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Rabbani F, Yazdiniapour Z, Ghanadian M, Zolfaghari B, Maleki M, Shafiee F. Cytotoxicity and apoptosis assay of novel cyclomyrsinol diterpenes against breast cancer cell lines. World J Tradit Chin Med. 2022;8:273–7.
Wu WX, Zhang M. Effect of baseline lipid level on prognosis of patients with non-triple-negative breast cancer. Chin Gen Pract. 2021;24(26):3316.
Gao Y, Wei W, Zhang P, Zhang Q. Advances in the study of natural history and tumor growth rate of breast cancer. Chin Gen Pract. 2021;24(30):3794.
Yuan KX, Wang JN. Construction and functional study of TUFT1 eukaryotic expression plasmid. J West Anhui Univ. 2017;33(2):71–4.
Shilo D, Cohen G, Blumenfeld A, Goren K, Hanhan S, Sharon S, et al. Tuftelin is required for NGF-induced differentiation of PC12 cells. J Mol Neurosci. 2019;68(1):135–43.
Wang K, Zhang YP, Yang YP. Expression of TUFT1 in rectal cancer and its clinical diagnostic and prognostic value. Chin J Lab Diagn. 2022;26(12):1788–92.
Zhou B, Lei JH, Wang Q, Qu TF, Cha LC, Zhan HX, et al. LINC00960 regulates cell proliferation and glycolysis in pancreatic cancer through the miR-326-3p/TUFT1/AKT-mTOR axis. Kaohsiung J Med Sci. 2022;38(12):1155–67.
Dou C, Zhou Z, Xu Q, Liu Z, Zeng Y, Wang Y, et al. Hypoxia-induced TUFT1 promotes the growth and metastasis of hepatocellular carcinoma by activating the Ca2+/PI3K/AKT pathway. Oncogene. 2019;38(8):1239–55.
Orgaz JL, Herraiz C, Sanz-Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5: e29019.
Baugher PJ, Krishnamoorthy L, Price JE, Dharmawardhane SF. Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells. Breast Cancer Res. 2005;7:R965–74.
Velaithan R, Kang J, Hirpara JL, Loh T, Goh BC, Le Bras M, et al. The small GTPase Rac1 is a novel binding partner of Bcl-2 and stabilizes its antiapoptotic activity. Blood. 2011;117:6214–26.
Shin SK, Ryu S, Nam S, Ha SY, Kwon OS, Kim YS, et al. Clinical significance of combined epithelial-mesenchymal transition markers expression and role of Rac1 in hepatocellular carcinoma. Int J Mol Sci. 2023;24(2):1765.
Acknowledgements
We would like to acknowledge the hard and dedicated work of all the staff who implemented the intervention and evaluation components of the study.
Funding
2021 Hebei Medical science research project plan (No.20210206).
Author information
Authors and Affiliations
Contributions
Sufang Shi: Conception and design of the research; Obtaining financing. Ruixi Cai: Conception and design of the research; Acquisition of data. Yong Li: Acquisition of data; Analysis and interpretation of the data. Yanfei Ren: Acquisition of data; Analysis and interpretation of the data. Shuo Li: Writing of the manuscript; Statistical analysis. Tianlu Yin: Writing of the manuscript; Statistical analysis. Weiguang Liu: Critical revision of the manuscript for intellectual content; Statistical analysis. Yongjun Li: Conception and design of the research; Critical revision of the manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was conducted with approval from the Ethics Committee of the Affiliated Hospital of Hebei Engineering University (No.2020[k]033). This study was conducted in accordance with the declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Sf., Cai, Rx., Ren, Yf. et al. Assessment of TUFT1 and Rac1-GTP levels in triple-negative breast cancer patients: clinical and pathological correlations. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03426-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03426-3