Abstract
Purpose
A previous real-world study conducted in China confirmed that first-line atezolizumab, in combination with etoposide/platinum (EP), leads to significantly longer progression-free survival (PFS) compared to EP alone in patients with extensive-stage small-cell lung cancer (ES-SCLC). The present study aimed to provide updated survival outcome data and evaluate the clinical efficacy of atezolizumab plus chemotherapy in ES-SCLC patients with brain metastasis (BM).
Methods
This retrospective study included 225 patients with ES-SCLC who were treated with EP alone (EP group) or a combination of EP + atezolizumab (atezolizumab group). Survival outcomes for the total study sample and patients in the BM subgroup were estimated using the Kaplan–Meier method.
Results
The atezolizumab group continued to demonstrate significantly longer PFS than the EP group (hazard ratio [HR], 0.68). The median overall survival (OS) was 26.2 months in the atezolizumab group vs. 14.8 months in the EP group (HR, 0.63). Additionally, among the BM patients in our study, the median PFS was found to be longer in the atezolizumab group (7.0 months) than in the EP group (4.1 months) (HR, 0.46). The OS of the BM patients did not differ significantly between the two treatment groups.
Conclusions
The addition of atezolizumab to EP as a first-line treatment for ES-SCLC was found to improve survival outcomes. This treatment combination may also prolong PFS in patients with BM, regardless of the administration of cranial irradiation. However, among the BM patients in our study, there was no significant difference in OS between the two treatment groups.




Similar content being viewed by others
Data availability
The data are available from the corresponding author by reasonable request.
References
American Cancer Society Lung cancer survival rates: 5-year survival rates for lung cancer. https://www.cancer.org/cancer/types/lung-cancer/detection-diagnosis-staging/survival-rates.html
Rittberg R, Banerji S, Kim JO, Rathod S, Dawe DE. Treatment and prevention of brain metastases in small cell lung cancer. Am J Clin Oncol. 2021;44:629–38.
Sundstrøm S, Bremnes RM, Kaasa S, Aasebø U, Hatlevoll R, Dahle R, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol. 2002;20:4665–72.
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–9.
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–39.
The Chinese Society of Clinical Oncology Clinical guidelines for the diagnosis and treatment of small-cell lung cancer
Chen H, Ma X, Liu J, Yang Y, Fang Y, Wang L, et al. Clinical outcomes of atezolizumab in combination with etoposide/platinum for treatment of extensive-stage small-cell lung cancer: a real-world, multicenter, retrospective, controlled study in China. Chin J Cancer Res. 2022;34:353–64.
Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. 2021;39:619–30.
Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22:51–65.
Elegbede AA, Gibson AJ, Fung AS, Cheung WY, Dean ML, Bebb DG, et al. A real-world evaluation of atezolizumab plus platinum-etoposide chemotherapy in patients with extensive-stage SCLC in Canada. JTO Clin Res Rep. 2021;2: 100249.
Lee S, Shim HS, Ahn BC, Lim SM, Kim HR, Cho BC, et al. Efficacy and safety of atezolizumab, in combination with etoposide and carboplatin regimen, in the first-line treatment of extensive-stage small-cell lung cancer: a single-center experience. Cancer Immunol Immunother. 2022;71:1093–101.
Schlick B, Shields MD, Marin-Acevedo JA, Patel I, Pellini B. Immune checkpoint inhibitors and chemoradiation for limited-stage small cell lung cancer. Curr Treat Options Oncol. 2022;23:1104–20.
NCCN Guidelines Version 3.2023. https://www.nccn.org/guidelines/recently-published-guidelines
Sun A, Abdulkarim B, Blais N, Greenland J, Louie AV, Melosky B, et al. Use of radiation therapy among patients with extensive-stage Small-cell lung cancer receiving Immunotherapy: Canadian consensus recommendations. Lung Cancer. 2023;179: 107166.
Li L, Yang D, Min Y, Liao A, Zhao J, Jiang L, et al. First-line atezolizumab/durvalumab plus platinum-etoposide combined with radiotherapy in extensive-stage small-cell lung cancer. BMC Cancer. 2023;23:318.
Liu C, Zeng L, Deng C, Jiang W, Wang Y, Zhou Y, et al. Hypofractionated radiotherapy with immunochemotherapy for extensive-stage small-cell lung cancer. Front Immunol. 2023;14:1175960.
Zhou L, Sun J, Xie C, Gong Y, Huang M, Yuan Z et al. Efficacy and safety of low-dose radiotherapy (LDRT) concurrent atezolizumab plus chemotherapy as first-line therapy for ES-SCLC: interim analysis of Phase II MATCH trial. J Clin Oncol. 2022;40:e20611-e
Galuba JM, Stöver I, Koziorowski A, Bölükbas S, Nilius G, Christoph DC. 1652P Safety of simultaneously performed radiotherapy in patients with small cell lung cancer undergoing atezolizumab treatment. Ann Oncol. 2021;32:S1165.
Bozorgmehr F, Christopoulos P, Chung I, Cvetkovic J, Feißt M, Krisam J, et al. Protocol of the TREASURE study: Thoracic RadiothErapy with Atezolizumab in Small cell lUng canceR Extensive disease – a randomized, open-label, multicenter phase II trial. BMC Cancer. 2022;22:1011.
Bozorgmehr F, Weykamp F, Overbeck TR, Maguire N, Buchmeier EL, Hammer-Hellmig M, et al. 1988MO recruitment discontinuation in TREASURE trial (thoracic radiotherapy with atezolizumab in small cell lung cancer extensive disease) due to unexpected safety data. Ann Oncol. 2023;34:S1060.
Yamamoto K, Hirano K, Ninomaru T, Okada H, Shimada T, Hata A. EP13.07-02 is concept of continuous immunotherapy beyond progression effective in small cell lung cancer? J Thorac Oncol. 2023;18:S706.
Li L, Liu T, Liu Q, Mu S, Tao H, Yang X, et al. Rechallenge of immunotherapy beyond progression in patients with extensive-stage small-cell lung cancer. Front Pharmacol. 2022;13:967559.
Ma J, Tian Y, Hao S, Zheng L, Hu W, Zhai X, et al. Outcomes of first-line anti-PD-L1 blockades combined with brain radiotherapy for extensive-stage small-cell lung cancer with brain metastasis. J Neurooncol. 2022;159:685–93.
Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–41.
van Bussel MTJ, Beijnen JH, Brandsma D. Intracranial antitumor responses of nivolumab and ipilimumab: a pharmacodynamic and pharmacokinetic perspective, a scoping systematic review. BMC Cancer. 2019;19:519.
Kowalski ES, Remick JS, Sun K, Alexander GS, Khairnar R, Morse E, et al. Immune checkpoint inhibition in patients treated with stereotactic radiation for brain metastases. Radiat Oncol. 2020;15:245.
Scoccianti S, Olmetto E, Pinzi V, Osti MF, Di Franco R, Caini S, et al. Immunotherapy in association with stereotactic radiotherapy for non-small cell lung cancer brain metastases: results from a multicentric retrospective study on behalf of AIRO. Neuro Oncol. 2021;23:1750–64.
Acknowledgements
This work was supported by National Natural Science Foundation of China (82141117); The Capital’s Funds for Health Improvement and Research (2022-2-1023); The Beijing Municipal Administration of Hospitals Incubating Program (Code: PX2020045); Wu Jieping Medical Foundation (320.6750.2021-16-19), Clinical Research Fund for Distinguished Young Scholars of Beijing Cancer Hospital (QNJJ2022012); Guangzhou Life Oasis Public Service Center Research and exchange program in the field of health (1-35).
Author information
Authors and Affiliations
Contributions
Conceptualization: Jian Fang, Jun Zhao, Minglei Zhuo; Methodology: Jie Liu, Jun Zhao, Minglei Zhuo; Formal analysis and investigation: Hanxiao Chen, Yu Yang, Yanhui He, Jian Fang; Data curation: Hanxiao Chen, Xiangjuan Ma, Jie Liu, Yu Yang, Yanhui He, Yong Fang, Liping Wang; Writing—original draft preparation: Hanxiao Chen, Yanhui He; Writing—review and editing: Hanxiao Chen, Xiangjuan Ma, Jian Fang, Jun Zhao, Minglei Zhuo; Funding acquisition: Jian Fang, Jun Zhao, Minglei Zhuo; Resources: Jian Fang; Supervision: Jun Zhao, Minglei Zhuo; Project administration: Hanxiao Chen, Jie Liu; Software: Hanxiao Chen, Xiangjuan Ma; Validation: Minglei Zhuo; Visualization: Minglei Zhuo.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Cancer Hospital (NO. 2021YJZ33-GZ01).
Informed consent
Informed consent was waived by the Ethics Committee because of the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12094_2024_3387_MOESM1_ESM.tif
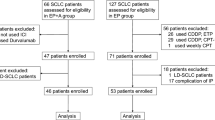
Supplementary file1 Online Resource 1. Eligibility and analysis. ES-SCLC, extensive-stage small-cell lung cancer. LS-SCLC, Limited-stage small-cell lung cancer. BM, brain metastasis (TIF 270 KB)
12094_2024_3387_MOESM2_ESM.tif
Supplementary file2 Online Resource 2. Kaplan–Meier analysis of PFS (A) and OS (C) in patients (chemosensitive subgroup) treated with EP + A compared with those treated with EP alone from the first cycle to at least 4 cycles in induction therapy. PFS: HR 0.58 (95% CI 0.41–0.83), P = 0.002; aHR 0.47 (95% CI 0.32–0.68), P < 0.001; OS: HR 0.53 (0.31–0.88), P = 0.015; aHR 0.46 (95% CI 0.26–0.80), P = 0.007. The figure also presents the results of Kaplan–Meier analysis of PFS (B) and OS (D) in patients (maintenance subgroup) treated with EP + A from the first cycle to at least 4 cycles in induction therapy and at least 1 cycle of atezolizumab in maintenance therapy compared with those treated with EP alone from the first cycle to at least 4 cycles in induction therapy. PFS: HR 0.43 (95% CI 0.28–0.65), P < 0.001; aHR 0.35 (95% CI 0.22–0.55), P < 0.001. OS: HR 0.40 (0.21–0.73), P = 0.004; aHR 0.40 (0.20–0.74), P = 0.005 (TIF 782 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, H., Ma, X., Liu, J. et al. Real-world evaluation of first-line treatment of extensive-stage small-cell lung cancer with atezolizumab plus platinum/etoposide: a focus on patients with brain metastasis. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03387-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03387-7