Abstract
Background
Gastric cancer (GC) is a malignancy that belongs to one of the most common leading causes of cancer death. Cancer-associated fibroblasts (CAFs) promote the GC cells’ malignant behavior. It is still unknown how GC converts normal fibroblasts (NFs) to CAFs.
Methods
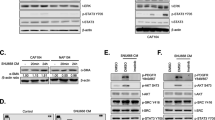
GC cells were co-cultured with NFs. Bioinformatics was used to analyze the genes and signaling pathways that were changed in fibroblast. RT-PCR, western blot, and Elisa assays were used to detect the expression of cytokines in fibroblast and condition medium. Western blot and immunofluorescence demonstrated activation of relevant pathways in CAFs-like cells. Transwell, scrape, colony formation, and CCK-8 assays were performed to reveal the feedback effect of CAFs-like cells on GC cells.
Results
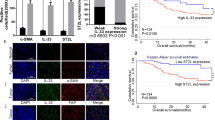
GC promoted the conversion of NFs to CAFs by secreting Interleukin 17A (IL-17). It included both morphological and molecular marker changes. This process was achieved by activating the nuclear factor-κB (NF-κB) pathway. On the other hand, CAFs cells could secrete C-X-C Motif Chemokine Ligand 8 (IL-8, IL-8), which promoted the malignant phenotype of GC cells. In this way, a feedback loop of mutual influence was constructed in the GC and tumor microenvironment (TME).
Conclusions
Our research proved a novel model of GC-educated NFs. GC-IL-17-fibroblast-IL-8-GC axis might be a potential pathway of the interaction between GC and TME.






Similar content being viewed by others
Data availability
No additional data are available.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA. 2021;71(1):7–33.
Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21114012.
Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Can Res. 2019;79(18):4557–66.
Zhang Y, Wang S, Lai Q, Fang Y, Wu C, Liu Y, et al. Cancer-associated fibroblasts-derived exosomal miR-17–5p promotes colorectal cancer aggressive phenotype by initiating a RUNX3/MYC/TGF-β1 positive feedback loop. Cancer Lett. 2020;491:22–35. https://doi.org/10.1016/j.canlet.2020.07.023.
Virchow R. As based upon physiological and pathological histology. Nutr Rev. 1989;47(1):23–5.
Tarin D, Croft C. Ultrastructural features of wound healing in mouse skin. J Anat. 1969;105(Pt 1):189–90.
Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401.
Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;99:186–96.
Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: cancer-associated fibroblasts and their markers. Int J Cancer. 2020;146(4):895–905. https://doi.org/10.1002/ijc.32193.
Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5(12):1640–6.
Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33(3):463-479. e.10.
Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer. 2020;19(1):43. https://doi.org/10.1186/s12943-020-01168-8.
Ishimoto T, Miyake K, Nandi T, Yashiro M, Onishi N, Huang KK, et al. Activation of transforming growth factor beta 1 signaling in gastric cancer-associated fibroblasts increases their motility, via expression of rhomboid 5 homolog 2, and ability to induce invasiveness of gastric cancer cells. Gastroenterology. 2017;153(1):191-204.e116. https://doi.org/10.1053/j.gastro.2017.03.046.
Wang X, Zhou Q, Yu Z, Wu X, Chen X, Li J, et al. Cancer-associated fibroblast-derived Lumican promotes gastric cancer progression via the integrin β1-FAK signaling pathway. Int J Cancer. 2017;141(5):998–1010. https://doi.org/10.1002/ijc.30801.
Liu X, Yao L, Qu J, Liu L, Lu N, Wang J, et al. Cancer-associated fibroblast infiltration in gastric cancer: the discrepancy in subtypes pathways and immunosuppression. J Transl Med. 2021;19(1):325. https://doi.org/10.1186/s12967-021-03012-z.
Itoh G, Chida S, Yanagihara K, Yashiro M, Aiba N, Tanaka M. Cancer-associated fibroblasts induce cancer cell apoptosis that regulates invasion mode of tumours. Oncogene. 2017;36(31):4434–44. https://doi.org/10.1038/onc.2017.49.
Karakasheva TA, Lin EW, Tang Q, Qiao E, Waldron TJ, Soni M, et al. IL-6 mediates cross-talk between tumor cells and activated fibroblasts in the tumor microenvironment. Cancer Res. 2018;78(17):4957–70. https://doi.org/10.1158/0008-5472.Can-17-2268.
Piao CH, Song CH, Lee EJ, Chai OH. Saikosaponin A ameliorates nasal inflammation by suppressing IL-6/ROR-γt/STAT3/IL-17/NF-κB pathway in OVA-induced allergic rhinitis. Chem Biol Interact. 2020;315:108874. https://doi.org/10.1016/j.cbi.2019.108874.
Zhang D, Li L, Jiang H, Li Q, Wang-Gillam A, Yu J, et al. Tumor-stroma IL1β-IRAK4 feedforward circuitry drives tumor fibrosis, chemoresistance, and poor prognosis in pancreatic cancer. Cancer Res. 2018;78(7):1700–12. https://doi.org/10.1158/0008-5472.Can-17-1366.
Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. https://doi.org/10.1016/j.immuni.2019.06.025.
Kuzet SE, Gaggioli C. Fibroblast activation in cancer: when seed fertilizes soil. Cell Tissue Res. 2016;365(3):607–19. https://doi.org/10.1007/s00441-016-2467-x.
Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36(13):1770–8. https://doi.org/10.1038/onc.2016.353.
Wen S, Hou Y, Fu L, Xi L, Yang D, Zhao M, et al. Cancer-associated fibroblast (CAF)-derived IL32 promotes breast cancer cell invasion and metastasis via integrin β3-p38 MAPK signalling. Cancer Lett. 2019;442:320–32. https://doi.org/10.1016/j.canlet.2018.10.015.
Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, et al. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8(14):3932–48. https://doi.org/10.7150/thno.25541.
Bertero T, Oldham WM, Grasset EM, Bourget I, Boulter E, Pisano S, et al. Tumor-stroma mechanics coordinate amino acid availability to sustain tumor growth and malignancy. Cell Metab. 2019;29(1):124-140.e110. https://doi.org/10.1016/j.cmet.2018.09.012.
Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. 2015;47(4):320–9. https://doi.org/10.1038/ng.3225.
Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554(7693):538–43. https://doi.org/10.1038/nature25492.
Tarrats N, Moles A, Morales A, García-Ruiz C, Fernández-Checa JC, Marí M. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology. 2011;54(1):319–27. https://doi.org/10.1002/hep.24388.
Pradere JP, Kluwe J, De Minicis S, Jiao JJ, Gwak GY, Dapito DH, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58(4):1461–73. https://doi.org/10.1002/hep.26429.
Bonnardel J, T’Jonck W, Gaublomme D, Browaeys R, Scott CL, Martens L, et al. Stellate cells, hepatocytes, and endothelial cells imprint the kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity. 2019;51(4):638-654.e639. https://doi.org/10.1016/j.immuni.2019.08.017.
Wu YH, Huang YF, Chang TH, Chen CC, Wu PY, Huang SC, et al. COL11A1 activates cancer-associated fibroblasts by modulating TGF-β3 through the NF-κB/IGFBP2 axis in ovarian cancer cells. Oncogene. 2021;40(26):4503–19. https://doi.org/10.1038/s41388-021-01865-8.
Rouvier E, Luciani M, Mattei M, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150(12):5445–56.
Hymowitz SG, Filvaroff EH, Yin J, Lee J, Cai L, Risser P, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001;20(19):5332–41.
Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–603.
Dragon S, Rahman MS, Yang J, Unruh H, Halayko AJ, Gounni AS. IL-17 enhances IL-1β-mediated CXCL-8 release from human airway smooth muscle cells. Am J Physiol-Lung Cell Mol Physiol. 2007;292(4):L1023–9.
Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281(34):24138–48.
Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–98.
Chen H, Wang W, Xie H, Xu X, Wu J, Jiang Z, et al. A pathogenic role of IL-17 at the early stage of corneal allograft rejection. Transpl Immunol. 2009;21(3):155–61.
Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, et al. The adaptor Act1 is required for interleukin 17–dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8(3):247–56.
El-Gedamy M, El-Khayat Z, Abol-Enein H, El-Said A, El-Nahrery E. Rs-10889677 variant in interleukin-23 receptor may contribute to creating an inflammatory milieu more susceptible to bladder tumourigenesis: report and meta-analysis. Immunogenetics. 2021;73(3):207–26. https://doi.org/10.1007/s00251-021-01205-w.
El-Gedamy M, El-Khayat Z, Abol-Enein H, El-Said A, El-Nahrery E. Rs-1884444 G/T variant in IL-23 receptor is likely to modify risk of bladder urothelial carcinoma by regulating IL-23/IL-17 inflammatory pathway. Cytokine. 2021;138:155355. https://doi.org/10.1016/j.cyto.2020.155355.
Zheng J, Jiang L, Zhang L, Yang L, Deng J, You Y, et al. Functional genetic variations in the IL-23 receptor gene are associated with risk of breast, lung and nasopharyngeal cancer in Chinese populations. Carcinogenesis. 2012;33(12):2409–16. https://doi.org/10.1093/carcin/bgs307.
Amatya N, Garg AV, Gaffen SL. IL-17 signaling: the yin and the yang. Trends Immunol. 2017;38(5):310–22.
Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, et al. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-κB and C/EBPβ activation. J Biol Chem. 2007;282(37):27229–38.
Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, et al. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci Signal. 2009;2(92):ra63.
Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988;167(5):1547–59.
Ha H, Debnath B, Neamati N. Role of the IL-8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics. 2017;7(6):1543.
Veltri RW, Miller MC, Zhao G, Ng A, Marley GM, Wright GL Jr, et al. Interleukin-8 serum levels in patients with benign prostatic hyperplasia and prostate cancer. Urology. 1999;53(1):139–47.
Zhang G, Gomes-Giacoia E, Dai Y, Lawton A, Miyake M, Furuya H, et al. Validation and clinicopathologic associations of a urine-based bladder cancer biomarker signature. Diagn Pathol. 2014;9(1):1–10.
Tong H, Ke J-Q, Jiang F-Z, Wang X-J, Wang F-Y, Li Y-R, et al. Tumor-associated macrophage-derived IL-8 could induce ERα suppression via HOXB13 in endometrial cancer. Cancer Lett. 2016;376(1):127–36.
New J, Arnold L, Ananth M, Alvi S, Thornton M, Werner L, et al. Secretory autophagy in cancer-associated fibroblasts promotes head and neck cancer progression and offers a novel therapeutic target. Cancer Res. 2017;77(23):6679–91. https://doi.org/10.1158/0008-5472.Can-17-1077.
Liubomirski Y, Lerrer S, Meshel T, Morein D, Rubinstein-Achiasaf L, Sprinzak D, et al. Notch-mediated tumor-stroma-inflammation networks promote invasive properties and IL-8 expression in triple-negative breast cancer. Front Immunol. 2019;10:804. https://doi.org/10.3389/fimmu.2019.00804.
Galbo PM Jr, Zang X, Zheng D. Molecular features of cancer-associated fibroblast subtypes and their implication on cancer pathogenesis, prognosis, and immunotherapy resistance. Clin Cancer Res. 2021;27(9):2636–47. https://doi.org/10.1158/1078-0432.Ccr-20-4226.
Hu H, Piotrowska Z, Hare PJ, Chen H, Mulvey HE, Mayfield A, et al. Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms. Cancer Cell. 2021;39(11):1531-1547.e1510. https://doi.org/10.1016/j.ccell.2021.09.003.
Funding
Liaoning S&T Project (2019-ZD-0592).
Author information
Authors and Affiliations
Contributions
All the authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the Faculty of Science Ethics Committee at Liaoning Cancer Hospital and Institute (20191215). All participants provided expressed consent for publication of their details. All personal information have been made anonymous.
Informed consent
All patients included in the study signed informed consent forms before surgery at the Liaoning Province Cancer Hospital and Institute.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, XK., Xie, B., Shao, Y. et al. Cytokine-driven positive feedback loop organizes fibroblast transformation and facilitates gastric cancer progression. Clin Transl Oncol 24, 1354–1364 (2022). https://doi.org/10.1007/s12094-022-02777-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02777-z