Abstract
Background
Incidence of gastric cancer (GC) shows different distribution in Italy, with higher incidence in the north and center. We retrospectively analyzed the clinical data of patients resected at the Hospital of Cremona between January 2007 and December 2016. Available clinical variables were linked with survival to identify possible prognostic factors.
Materials and methods
Variables analyzed were age, sex, type of surgery, site, histology, invasion, nodal status, resection margins, grade, HER2 status, Helicobacter pylori infection (neo)adjuvant chemotherapy, adjuvant chemoradiotherapy, neutrophil-to-lymphocyte ratio, number of nodes removed and type of lymphadenectomy. Overall survival (OS) was estimated by the Kaplan–Meier method and differences between groups by the log-rank test. Data on OS were analyzed by Cox regression and the final model was obtained using the step-wise method.
Results
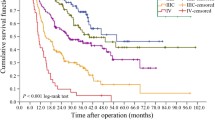
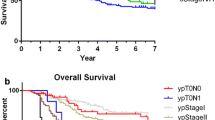
379 patients were considered, out of which 195 were operated from 2007 to 2011 and 184 from 2012 to 2016. Median follow-up was 25.5 months, median OS 31.3 months and time to recurrence 23.2 months. D2 resection rate increased from 36% (period 2007–2011) to 74% in 2012–2016 (p = 0.01) with a higher mean number of nodes collected (20.98 for 2007–2011 and 23.53 for 2012–2016, p = 0.040). Only 37% of patients received a postoperative treatment. At multivariate analysis, variables associated with OS were age (p = 0.002), stage (p < 0.001), resection margins status (p < 0.001), adjuvant chemotherapy (p < 0.010) and tumor location (cardia vs non-cardia) (p = 0.029).
Conclusions
Our analysis shows that completeness of resection and lower stage are strong predictors of long-term survival in GC, providing the rationale for adjuvant and neoadjuvant approaches (chemotherapy, radiotherapy or combined). Cardial GC has worse prognosis compared to distal cancers.
Trial registration number
Service evaluation number 256, protocol 16821/17, date 05 June 2017.


Similar content being viewed by others
References
World Health Organization. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide (Version 2012). https://globocan.iarc.fr/Default.aspx. Accessed August 14, 2018.
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403.
Associazione Italiana di Oncologia Medica. I numeri del cancro in Italia AIOM-AIRTUM (Version 2017). https://www.aiom.it/fondazione-aiom/aiom-airtum-numeri-cancro-2017/aiom-airtum-numeri-cancro-2017/130211. Accessed 14 Aug 2018.
Edwards BK, Noone AM, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–314.
Swan R, Miner TJ. Current role of surgical therapy in gastric cancer. World J Gastroenterol. 2006;12:372–9.
Schirren R, Reim D, Novotny AR. Adjuvant and/or neoadjuvant therapy for gastric cancer? A perspective review. Ther Adv Med Oncol. 2015;7:39–48.
Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635–49.
Union for International Cancer Control’s. 8th edition of the UICC TNM classification of malignant tumors (version 2018). https://www.uicc.org/8th-edition-uicc-tnm-classification-malignant-tumors-published. Accessed 14 Aug 2018.
Mocellin S, McCulloch P, Kazi H, et al. Extent of lymph node dissection for adenocarcinoma of the stomach. Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.CD001964.pub4.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, gastric cancer (version 2.2018). Accessed 14 Aug 2018.
Woo Y, Goldner B, Ituarte P, et al. Lymphadenectomy with optimum of 29 lymph nodes retrieved associated with improved survival in advanced gastric cancer: a 25,000-patient international database study. J Am Coll Surg. 2017;224:546–55.
Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697–708.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–21.
Petrelli F, Ghidini M, Barni S, et al. Prognostic role of primary tumor location in non-metastatic gastric cancer: a systematic review and meta-analysis of 50 studies. Ann Surg Oncol. 2017;24:2655–68.
Bria E, De Manzoni G, Beghelli S, et al. A clinical-biological risk stratification model for resected gastric cancer: prognostic impact of Her2, Fhit, and APC expression status. Ann Oncol. 2013;24:693–701.
Acknowledgements
Authors want to thank Dr. Michael Davies (Unique Language Training) for linguistic revision.
Funding
No grants supported this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflicts of interest to declare.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethics committee
Approval for this project was obtained before the start of the study from the local Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghidini, M., Donida, B.M., Totaro, L. et al. Prognostic factors associated with survival in a large cohort of gastric cancer patients resected over a decade at a single Italian center: the Cremona experience. Clin Transl Oncol 22, 1004–1012 (2020). https://doi.org/10.1007/s12094-019-02220-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02220-w