Abstract
Purpose
To describe patient characteristics by disease stage, resectability status and current treatment management after first diagnosis of IIIB to IV1c advanced (AM)/metastatic melanoma (MM).
Methods/patients
Multicentre, retrospective study based on data from medical charts of patients > 18 years at MM first diagnosis, visited by oncologists at 4 reference centres in Spain: Hospital Universitario Gregorio Marañón (Madrid), Hospital General de Valencia (Valencia), Clínica Universidad de Navarra (Pamplona), and Hospital Clínic (Barcelona).
Results
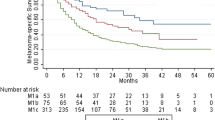
Metastatic non-visceral melanoma (IIIB, IIIC, IV M1a) was reported in 139 (48.6%) patients and 40.9% (n = 117) were diagnosed with IV-M1c disease. 160 (55.9%) metastases were resectable. Available therapies under clinical practice were used in 210 patients; 74 were treated under clinical trials (CT). Intention-to-cure surgery (47.6%) was the most common treatment at time of MM diagnosis. Systemic (45.1% overall) therapy included chemo-, targeted- and immunotherapy (19.6%, 14.3%, 8.4%, respectively). At time of data collection, 26 patients were still alive and 120 had progressed to IV-M1c. Median overall survival (OS) was significantly larger in IIIB patients, 28.9 m (25.2–32.7); the shortest for IV-M1c patients, 11.0 m (8.7–13.3).
Conclusions
Novel treatments are undoubtedly a major step forward in AM/MM, however these are often only available in the CT setting because early stages of development or country-specific regulations. Further prospective studies and multifactorial analysis should be performed to clearly identify possible clinical associations for outcome in Spanish patients with AM/MM.


Similar content being viewed by others

References
Dummer R. Integrating first-line treatment options into clinical practice: what’s new in advanced melanoma? Melanoma Res. 2015;25:461–9.
Berrocal A, Arance A, Castellón VE, de la Cruz L, Espinosa E, Cao MG, et al. SEOM clinical guideline for the management of malignant melanoma (2017). Clin Transl Oncol. 2018;20:69–74.
Garbe C. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline—update 2016. Eur J Cancer. 2016;63:201–17.
Ugurel S. Survival of patients with advanced metastatic melanoma: the impact of novel therapies—update 2017. Eur J Cancer. 2017;83:247–57.
Polkowska M. Survival of melanoma patients treated with novel drugs: retrospective analysis of real-world data. J Cancer Res Clin Oncol. 2017;143:2087–94.
Martín-Algarra S. Guidelines for biomarker testing in metastatic melanoma: a National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin Transl Oncol. 2014;16:362–73.
Avilés JA. Epidemiology and survival of cutaneous melanoma in Spain: a report of 552 cases (1994–2003). Rev Clin Esp. 2006;206:319–25.
Sáenz S. Melanoma epidemiology in Spain. Actas Dermosifiliogr. 2005;96:411–8.
Ríos L, Nagore E, López JL, Redondo P, Martí RM, Fernández-de-Misa R, et al. Melanoma characteristics at diagnosis from the Spanish national cutaneous melanoma registry: 15 years of experience. Actas Dermosifiliogr. 2013;104:789–99.
Mohr PM, Ascierto P, Arance A, McArthur G, Hernaez A, Kaskel P, et al. Real-world treatment patterns and outcomes among metastatic cutaneous melanoma patients treated with ipilimumab. J Eur Acad Dermatol Venereol. 2017;71(5).
Jochems A, Schouwenburg MG, Leeneman B, Franken G, van den Eertwegh AJ, Haanen JB, et al. Dutch Melanoma Treatment Registry: quality assurance in the care of patients with metastatic melanoma in the Netherlands. Eur J Cancer. 2017;72:156–65.
Moreno-Ramírez D, de la Cruz L, Ferrándiz L, Camacho FM. Study and treatment of locally advanced melanoma. Actas Dermosifiliogr. 2009;100:767–79.
Dummer R, Hauschild A, Lindenblatt N, Pentheroudakis G, Keilholz U on behalf of the ESMO Guidelines Committee. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015; 5:v126–132.
European Medicines Agency. EPAR summary for the public. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Summary_for_the_public/human/002409/WC500124320.pdf.
Agencia Española de Medicamentos y Productos Sanitarios. Informe de Posicionamiento terapeutico. PT/V1/15112013,2. Informe de Posicionamiento Terapéutico de Vemurafenib (Zelboraf®). https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT-zelboraf-melanoma.pdf.
Diamantopoulos P, Gogas H. Melanoma immunotherapy dominates the field. Ann Transl Med. 2016;4:269.
Murrell J, Board R. The use of systemic therapies for the treatment of brain metastases in metastatic melanoma: opportunities and unanswered questions. Cancer Treat Rev. 2013;39:833–8.
Harries M, Malvehy J, Lebbe C, Heron L, Amelio J, Szabo Z, et al. Treatment patterns of advanced malignant melanoma (stage III–IV)—a review of current standards in Europe. Eur J Cancer. 2016;60:179–89.
Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. JCO. 2018;36:1658–67.
Salvador J, Urtasun JA, Duart FJB, García-Campelo R, Carbonero RG, Lianes P, et al. Equity, barriers and cancer disparities: study of the Spanish Society of Medical Oncology on the access to oncologic drugs in the Spanish Regions. Clin Transl Oncol. 2017;19:341–56.
A. Percentage of patients treated under available therapies or within a clinical trial (RCT) according to stage first-treatment line treatment for advanced melanoma (real clinical practice or as part of a RCT) from 2007 to 2015.
Acknowledgements
This study was funded by Amgen SA. Medical writing support was provided Cindy L. Larios M.D. from MFAR Clinical Research S.L. (Spain).
Funding
Medical writing support for this manuscript was funded by Amgen SA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Iván Márquez Rodas has participated in consulting and advisory boards of BMS, MSD, Novartis, Roche, Pierre-Fabre, Amgen, AstraZeneca, Merck-Serono, Incyte, Bioncotech, and Sanofi. Ana Arance has participated in consulting, advisory boards and Speaker’s bureau of BMS, Roche, MSD and Novartis. Alfonso Berrocal has received honoraria for consulting and advisory boards of MSD, BMS, Roche and Novartis. Cindy L. Larios and Jordi Curto are employed by MFAR Clinical Research, a CRO for Amgen for this study, analysis and manuscript. Ignasi X. Campos Tapias and Ana Belén Blanca are full-time employees at Amgen S.A. and own Amgen stocks. Salvador Martín Algarra reports receiving advisory board fees from Amgen, BMS, Incyte, Merck-Serono, MSD, Pierre-Fabre, Tesaro and Roche; speaker activities fees from BMS, MSD and Pierre-Fabre; consulting fees from Novartis and travel support from Roche.
Ethical approval
The study was approved by an independent Ethical Committee and conducted in accordance with the good clinical practice (GCP), the principles of the Declaration of Helsinki and current local applicable regulations.
Informed consent
All patients participating in the study signed informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Márquez-Rodas, I., Arance, A., Berrocal, A. et al. A retrospective chart review study describing metastatic melanoma patients profile and treatment patterns in Spain. Clin Transl Oncol 21, 1754–1762 (2019). https://doi.org/10.1007/s12094-019-02201-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02201-z