Abstract
Background
KRAS mutations are prevalent in non-small cell lung cancer (NSCLC) but its clinical implications remain to be determined. Continual profiling of KRAS mutations in patients is challenging, and the study aims to determine the potential use of urinary DNA in disease predictions.
Methods
A total of 150 patients were recruited. To ascertain the clinical relevance of urinary DNA, matched tumor profiles were analyzed. Serial measurements were taken to gauge the reliability of the assay. These results were correlated to overall survival using the Kaplan–Meier estimate.
Results
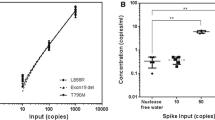
A good overall concordance of 93% (consolidated results from serial measurements) was achieved between tumor tissue and urinary DNA profiling. Of the discordant KRAS cases, we observed subsequent positive detection during monitoring and very low concentrations of mutant DNA. In addition, we noted that KRAS-positive patients detected using urinary DNA have good prognostic utility. Interestingly, we also observed that the trend is highly correlative of the rate of change in KRAS mutant DNA concentrations and the period of monitoring.
Conclusions
Urinary DNA offered a non-invasive approach to probe NSCLC dynamics, and in our study we showed that it had predictive capabilities for KRAS-positive patients. Serial monitoring of urinary samples showed that it had a predictive role in identifying patients with worse outcome.




Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917.
Chang A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung cancer. 2011;71(1):3–10.
Li T, Kung H-J, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol. 2013;31(8):1039–49.
Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, et al. The case for early detection. Nat Rev Cancer. 2003;3(4):243–52.
Brinkmann B, Rand S, Bajanowski T. Forensic identification of urine samples. Int J Legal Med. 1992;105(1):59–61.
Roberts PJ, Stinchcombe TE, Der CJ, Socinski MA. Personalized medicine in non-small-cell lung cancer: is KRAS a useful marker in selecting patients for epidermal growth factor receptor–targeted therapy? J Clin Oncol. 2010;28(31):4769–77.
Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486(7404):532–6.
Krypuy M, Newnham GM, Thomas DM, Conron M, Dobrovic A. High resolution melting analysis for the rapid and sensitive detection of mutations in clinical samples: KRAS codon 12 and 13 mutations in non-small cell lung cancer. BMC Cancer. 2006;6(1):295.
J-l Guan, W-z Zhong, S-j An, Yang J-j SuJ, Z-h Chen, et al. KRAS mutation in patients with lung cancer: a predictor for poor prognosis but not for EGFR-TKIs or chemotherapy. Ann Surg Oncol. 2013;20(4):1381–8.
Mukhopadhyay S. Utility of small biopsies for diagnosis of lung nodules: doing more with less. Mod Pathol. 2012;25:S43–57.
Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12(5):323–34.
Meyer JE, Smith DN, Lester SC, DiPiro PJ, Denison CM, Harvey SC, et al. Large-needle core biopsy: nonmalignant breast abnormalities evaluated with surgical excision or repeat core biopsy. Radiology. 1998;206(3):717–20.
Pathak AK, Bhutani M, Kumar S, Mohan A, Guleria R. Circulating cell-free DNA in plasma/serum of lung cancer patients as a potential screening and prognostic tool. Clin Chem. 2006;52(10):1833–42.
Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500.
Szarvas T, Kovalszky I, Bedi K, Szendroi A, Majoros A, Riesz P, et al. Deletion analysis of tumor and urinary DNA to detect bladder cancer: urine supernatant versus urine sediment. Oncol Rep. 2007;18(2):405–10.
Anker P, Mulcahy H, Chen XQ, Stroun M. Detection of circulating tumour DNA in the blood (plasma/serum) of cancer patients. Cancer Metastasis Rev. 1999;18(1):65–73.
Yokota M, Tatsumi N, Tsuda I, Takubo T, Hiyoshi M. DNA extraction from human urinary sediment. J Clin Lab Anal. 1998;12(2):88–91.
Botezatu I, Og Serdyuk, Potapova G, Shelepov V, Alechina R, Molyaka Y, et al. Genetic analysis of DNA excreted in urine: a new approach for detecting specific genomic DNA sequences from cells dying in an organism. Clin Chem. 2000;46(8):1078–84.
Chan BA, Hughes BGM. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4(1):36.
Metro G, Chiari R, Duranti S, Siggillino A, Fischer MJ, Giannarelli D, et al. Impact of specific mutant KRAS on clinical outcome of EGFR-TKI-treated advanced non-small cell lung cancer patients with an EGFR wild type genotype. Lung Cancer. 2012;78(1):81–6.
Santos E, Martin-Zanca D, Reddy EP, Pierotti MA, Della Porta G, Barbacid M. Malignant activation of a K-ras oncogene in lung carcinoma but not in normal tissue of the same patient. Science. 1984;223(4637):661–4.
Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359(4):366–77. doi:10.1056/NEJMoa0800668.
Goto K, Ichinose Y, Ohe Y, Yamamoto N, Negoro S, Nishio K, et al. Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J Thorac Oncol. 2012;7(1):115–21.
Kimura H, Suminoe M, Kasahara K, Sone T, Araya T, Tamori S, et al. Evaluation of epidermal growth factor receptor mutation status in serum DNA as a predictor of response to gefitinib (IRESSA). Br J Cancer. 2007;97(6):778–84. doi:10.1038/sj.bjc.6603949.
Marchetti A, Del Grammastro M, Felicioni L, Malatesta S, Filice G, Centi I, et al. Assessment of EGFR mutations in circulating tumor cell preparations from NSCLC patients by next generation sequencing: toward a real-time liquid biopsy for treatment. PLoS One. 2014;9(8):e103883.
Punnoose EA, Atwal SK, Spoerke JM, Savage H, Pandita A, Yeh R-F, et al. Molecular biomarker analyses using circulating tumor cells. PLoS One. 2010;5(9):e12517.
Earl J, Garcia-Nieto S, Martinez-Avila JC, Montans J, Sanjuanbenito A, Rodríguez-Garrote M, et al. Circulating tumor cells (Ctc) and KRAS mutant circulating free Dna (cfdna) detection in peripheral blood as biomarkers in patients diagnosed with exocrine pancreatic cancer. BMC Cancer. 2015;15(1):797.
Turke AB, Zejnullahu K, Wu Y-L, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17(1):77–88.
Yoon HJ, Lee HY, Lee KS, Choi Y-L, Ahn M-J, Park K, et al. Repeat biopsy for mutational analysis of non-small cell lung cancers resistant to previous chemotherapy: adequacy and complications. Radiology. 2012;265(3):939–48.
Cerfolio RJ, Bryant AS, Winokur TS, Ohja B, Bartolucci AA. Repeat FDG-PET after neoadjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg. 2004;78(6):1903–9.
Wang X, Meng Q, Wang C, Li F, Zhu Z, Zhu Z, Liu S, et al. Investigation of transrenal KRAS mutation in late stage NSCLC patients correlates to disease progression. Biomark: Biochem Indic Expo Res Susceptibility Chem. 2016;. doi:10.1080/1354750X.2016.1269202.
García-Saenz JA, Ayllón P, Laig M, Acosta-Eyzaguirre D, García-Esquinas M, Montes M, et al. Tumor burden monitoring using cell-free tumor DNA could be limited by tumor heterogeneity in advanced breast cancer and should be evaluated together with radiographic imaging. BMC Cancer. 2017;17(1):210.
Richardson AL, Iglehart JD. BEAMing up personalized medicine: mutation detection in blood. Clin Cancer Res. 2012;18(12):3209–11.
Acknowledgements
This work was funded by a research grant provided by the Yangtze University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Patients were recruited after providing informed consent to participate in the trial. The recruitment process and sample extractions followed strictly the guidelines approved by the institutional review board (IRB).
Rights and permissions
About this article
Cite this article
Xie, F., Li, P., Gong, J. et al. Urinary cell-free DNA as a prognostic marker for KRAS-positive advanced-stage NSCLC. Clin Transl Oncol 20, 591–598 (2018). https://doi.org/10.1007/s12094-017-1754-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1754-7