Abstract
Background
The role of bevacizumab in metastatic breast cancer is controversial. Identification of predictive biomarkers could help to select patients who really benefit from it. We evaluated the association of angiogenesis-related gene polymorphisms with the treatment outcome of bevacizumab in metastatic breast cancer patients.
Patients and methods
eNOS-786T/C and -894G/T, IL-8-251T/A genomic polymorphisms were assessed in 31 metastatic breast cancer patients treated with bevacizumab plus chemotherapy in the first-line setting. Testing for association between each polymorphism and treatment outcome was performed.
Results
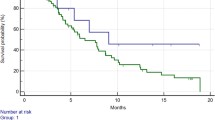
Patients with IL-8 251 AA genotype showed a significantly lower progression-free survival in each combination comparison: “TT” vs “AA” (13 vs 8 months; p = 0.008); TT vs TA vs AA (13 vs 11 vs 8 months; p = 0.02); TT vs TA +AA (13 vs 11 months; p = 0.01); TT + TA vs AA (12 vs 8 months; p = 0.01) and a lower overall survival when compared with TT +TA genotype (26 vs 51 months, p = 0.04). Patients carrying eNOS 894 TT genotype showed a statistically significant lower progression-free survival than patients with GG genotype (11.5 vs 26.5 months; p = 0.04) with no differences in the overall survival. No association with response rate was found with any of the polymorphisms analyzed.
Conclusion
These findings suggest that IL-8 251T/A and eNOS-894 G/T polymorphisms might have a role in predicting treatment outcome of bevacizumab in metastatic breast cancer. Our results are hypothesis generating and need to be confirmed in larger clinical trials.


Similar content being viewed by others
References
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23(15):3502–8.
Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–11.
Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–96.
Miles DW. Bevacizumab in breast cancer: fundamental questions remain. Lancet Oncol. 2013;14(2):99–101. doi:10.1016/S1470-2045(13)70012-3.
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76.
Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL. Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2009;27:4966–72. doi:10.1200/JCO.2008.21.6630.
Miles DW, Chan A, Dirix LY, Cortés J, Pivot X, Tomczak P, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–47. doi:10.1200/JCO.2008.21.6457.
Robert NJ, Dieras V, Glaspy J, Brufsky A, Bondarenko I, Lipatov O, et al. RIBBON-1:randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (MBC) [abstract]. J Clin Oncol. 2009;27:s15.
O’Shaughnessy JA, Brufsky AM. RiBBON 1 and RiBBON 2: phase III trials of bevacizumab with standard chemotherapy for metastatic breast cancer. Clin Breast Cancer. 2008;8(4):370–3. doi:10.3816/CBC.2008.n.045.
Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2011;29(32):4286–93. doi:10.1200/JCO.2010.34.1255.
Smith IE, Pierga JY, Biganzoli L, Cortés-Funes H, Thomssen C, Pivot X, et al. First-line bevacizumab plus taxane-based chemotherapy for locally recurrent or metastatic breast cancer: safety and efficacy in an open-label study in 2251 patients. Ann Oncol. 2011;22(3):595–602. doi:10.1093/annonc/mdq430.
Cuppone F, Bria E, Vaccaro V, Puglisi F, Fabi A, Sperduti I, et al. Magnitude of risks and benefits of the addition of bevacizumab to chemotherapy for advanced breast cancer patients: Meta-regression analysis of randomized trials. J Exp Clin Cancer Res. 2011; doi:10.1186/1756-9966-30-54.
Miles DW, Diéras V, Cortés J, Duenne AA, Yi J, O’Shaughnessy J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol. 2013 [Epub ahead of print].
Valachis A, Polyzos NP, Patsopoulos NA, Georgoulias V, Mavroudis D, Mauri D. Bevacizumab in metastatic breast cancer: a meta-analysis of randomized controlled trials. Breast Cancer Res Treat. 2010;122(1):1–7. doi:10.1007/s10549-009-0727-0.
Rose S. FDA pulls approval for avastin in breast cancer. Cancer Discov. 2011;1(7):OF1–2. doi:10.1158/2159-8290.CD-ND112311OL-08.
Maru D, Venook AP, Ellis LM. Predictive biomarkers for bevacizumab: are we there yet? Clin Cancer Res. 2013;19(11):2824–7. doi:10.1158/1078-0432.CCR-12-3409.
Miles DW, de Haas SL, Dirix LY, Romieu G, Chan A, Pivot X, et al. Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer. 2013;108(5):1052–60. doi:10.1038/bjc.2013.69.
Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–8.
Schneider BP, Gray RJ, Radovich M, Shen F, Vance G, Li L, et al. Prognostic and predictive value of tumor VEGF gene amplification in metastatic breast cancer treated with paclitaxel with and without bevacizumab; results from ECOG 2100 trial. Clin Cancer Res. 2013;19(5):1281–9. doi:10.1158/1078-0432.CCR-12-3029.
Kümler I, Christiansen OG, Nielsen DL. A systematic review of bevacizumab efficacy in breast cancer. Cancer Treat Rev. 2014;40(8):960–73.
Schultheis AM, Lurje G, Rhodes KE, Zhang W, Yang D, Garcia AA, et al. Polymorphisms and clinical outcome in recurrent ovarian cancer treated with cyclophosphamide and bevacizumab. Clin Cancer Res. 2008;14(22):7554–63. doi:10.1158/1078-0432.CCR-08-0351.
Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258(5089):1798–801.
Varney ML, Olsen KJ, Mosley RL, Bucana CD, Talmadge JE, Singh RK. Monocyte/macrophage recruitment, activation and differentiation modulate interleukin-8 production: a paracrine role of tumor-associated macrophages in tumor angiogenesis. In Vivo. 2002;16(6):471–7.
Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11(9):992–7.
Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, et al. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70(3):1063–71. doi:10.1158/0008-5472.CAN-09-3965.
Gyanchandani R, Sano D, Ortega Alves MV, Klein JD, Knapick BA, Oh S, Myers JN, et al. Interleukin-8 as a modulator of response to bevacizumab in preclinical models of head and neck squamous cell carcinoma. Oral Oncol. 2013;49(8):761–70. doi:10.1016/j.oraloncology.2013.03.452.
Abajo A, Boni V, Lopez I, Gonzalez-Huarriz M, Bitarte N, Rodriguez J, et al. Identification of predictive circulating biomarkers of bevacizumab-containing regimen efficacy in pre-treated metastatic colorectal cancer patients. Br J Cancer. 2012;107:287–90.
Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28:453–9.
Hautamaki a, Kivioja J, Vavuli S, Kakko S, Savolainen ER, Savolainen MJ et al. Interleukin 8 promoter polymorphism predicts the initial response to bevacizumab treatment for exudative age-related macular degeneration. Retina. 2013 [Epub ahead of print].
Hull J, Thomson A, Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000;55:1023–7.
Eechoute K, van der Veldt AA, Oosting S, Kappers MH, Wessels JA, Gelderblom H, et al. Polymorphisms in endothelial nitric oxide synthase (eNOS) and vascular endothelial growth factor (VEGF) predict sunitinib-induced hypertension. Clin Pharmacol Ther. 2012;92(4):503–10. doi:10.1038/clpt.2012.136.
Ando M. Nephrotoxicity—proteinuria and hypertension—. Gan To Kagaku Ryoho. 2008;35(10):1649–53.
Perlik M, Seremak-Mrozikiewicz A, Barlik M, Kurzawińska G, Kraśnik W, Drews K. Genetic variants of eNOS in gestational hypertension and pre-eclampsia. Ginekol Pol. 2012;83(9):652–9.
Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20(2):227–30. doi:10.1093/annonc/mdn637.
Smith KC, Bateman AC, Fussell HM, Howell WM. Cytokine gene polymorphism and breast cancer susceptibility and prognosis. Eur J Immunogenet. 2004;31:167–73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they do not have any conflict of interest.
Rights and permissions
About this article
Cite this article
Di Salvatore, M., Lo Giudice, L., Rossi, E. et al. Association of IL-8 and eNOS polymorphisms with clinical outcomes in bevacizumab-treated breast cancer patients: an exploratory analysis. Clin Transl Oncol 18, 40–46 (2016). https://doi.org/10.1007/s12094-015-1334-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1334-7