Abstract
Aim
Advanced pancreatic cancer has a bad prognosis, with a median overall survival (OS) no longer than 4–6 months. Since the end of last century, monotherapy with gemcitabine has remained the elective therapy, but new schedules are needed in order to improve these results. We aim to evaluate the efficacy of tegafur and levofolinic acid (LV) associated with gemcitabine, as well as its toxicity, progressionfree survival and OS in advanced pancreatic cancer.
Patients and methods
An open-label, multicentric, prospective, non-controlled trial was carried out on patients with advanced or disseminated pancreatic cancer. Gemcitabine 1250 mg/m2 was administered on the 1st and 8th days of the cycle, tegafur 750 mg/m2/day for 21 consecutive days and LV 25 mg/day continuously, every 28 days, with a maximum of six cycles. The primary variable was tumour overall response rate (ORR). Secondarily, time to progression (TTP), OS and scheme toxicity were determined.
Results
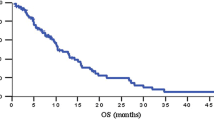
Forty patients were recruited; the male/female ratio was 30:10, with a mean age of 61 years. Forty percent had a Karnofsky index of 90% or 100%. Only 11 patients (27%) completed the six cycles of treatment, but more than 50% received three or more cycles. Dose intensity was 89.56% for gemcitabine and 87.36% for tegafur. Efficacy ORR was 22.5% (CI 95%, 6–37%). TTP was 3.87 months (CI 95%, 2.1–5.6), time to treatment failure was 2.97 months (CI 95%, 2.43–4.67) and OS 6.3 months (CI 95%, 4–7). The chemotherapeutic combination was well accepted; most haematologic and non-haematologic toxicities were grade 1 or 2. The most prevalent grade 3/4 toxicities were asthenia (30%), liver biochemistry disorders (25%), diarrhoea (15%) and stomatitis (12%).
Conclusions
The administration of gemcitabine, associated with oral tegafur and leucovorin, has activity against advanced pancreatic cancer, with an adequate toxicity profile.
Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Sultana A, Smith CT, Cunningham D et al (2007) Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 25:2607–2615
Glimelius B, Hoffman K, Sjoden PO et al (1996) Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 7:593–600
Burris HA III, Moore MJ, Andersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Maisey N, Chau I, Cunningham D et al (2002) Multicenter randomized phase III trial comparing protracted venous infusion (PVI) fluorouracil (5-FU) with PVI 5-FU plus mitomycin in inoperable pancreatic cancer. J Clin Oncol 20:3130–3136
Bedikian AY, Stroehlein J, Korinek J et al (1983) A comparative study of oral tegafur and intravenous 5-fluorouracil in patients with metastatic colorectal cancer. Am J Clin Oncol 6:181–186
Bjerkeset T, Fjosne HE (1986) Comparison of oral ftorafur and intravenous 5-fluorouracil in patients with advanced cancer of the stomach, colon or rectum. Oncology 43:212–215
Nogué M, Saigí E, Seguí MA (1995) Clinical experience with tegafur and low dose oral leucovorin: a dose-finding study. Oncology 52:167–169
Nogué M, Seguí MA, Saigí E et al (1998) Protracted treatment with tegafur and low dose oral leucovorin in patients with advanced colorectal carcinoma. 83:254–258
Nogué M, Salud A, Batiste-Alentorn E et al (2005) Randomised study of tegafur and oral leucovorin versus intravenous 5-fluorouracil and leucovorin in patients with advanced colorectal cancer. Eur J Cancer 41:2241–2249
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
National Cancer Institute (1999) Guidelines for the reporting of adverse drug reactions. Division of Cancer Treatment, National Cancer Institute, MD, pp 1–80
Van Cutsem E, Vervenne WL, Bennouna J et al (2009) Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 27:2231–2237
Hidalgo M, Abad A, Aranda E et al (2009) Consensus on the treatment of pancreatic cancer in Spain. Clin Transl Oncol 11:290–301
Heinemann V, Boeck S, Hinke A et al (2008) Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 8:82
Berlin JD, Catalano P, Thomas JP et al (2002) Phase III study of gemcitabine in combination with fluorouracil vs gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20:3270–3275
Riess H, Helm A, Niedergethmann M et al (2005) A randomised, prospective, multicentre, phase III trial of gemcitabine, 5-Fluorouracil, folinic acid vs gemcitabine alone in patients with advanced pancreatic cancer. J Clin Oncol 23[Suppl 16]:LBA4009
Di Costanzo F, Carlini P, Doni L et al (2005) Gemcitabine with or without continuous infusion 5-FU in advanced pancreatic cancer: a randomised phase II trial of the Italian oncology group for clinical research (GOIRC). Br J Cancer 93:185–189
Ohkawa S (2004) Randomised controlled trial of gemcitabine in combination with UFT vs gemcitabine alone in patients with advanced pancreatic cancer. J Clin Oncol 22[Suppl 14]:abstr LBA 4131
Louvet C, Labianca R, Hammel P et al (2005) Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 23:3509–3516
Herrmann R, Bodoky G, Ruhstaller T et al (2007) Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomised, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 25:2159–2161
Cunningham D, Chau I, Stocken DD et al (2009) Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 27:5513–5518
Moore MJ, Goldstein D, Hamm J et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960–1966
Philip PA, Benedetti J, Fenoglio-Preiser C et al (2007) Phase III study of gemcitabine [G] plus cetuximab [C] versus gemcitabine in patients [pts] with locally advanced or metastatic pancreatic adenocarcinoma [PC]: SWOG S0205 study. J Clin Oncol 25[Suppl 18]:abstr LBA4509
Kindler HL, Niedzwiecki D, Hollis D et al (2007) A double-blind, placebo-controlled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients (pts) with advanced pancreatic cancer (PC): a preliminary analysis of Cancer and Leukemia Group B (CALGB). J Clin Oncol 25[Suppl 18]:abstr 4508
Author information
Authors and Affiliations
Corresponding author
Additional information
on behalf of the ACROSS group
Rights and permissions
About this article
Cite this article
Pericay Pijaume, C., Escudero Emperador, P., Bastús Piulats, R. et al. Open-label trial on efficacy and security of treatment with gemcitabine and oral modulation with tegafur and levofolinic acid (GEMTG) in patients with advanced pancreatic cancer. Clin Transl Oncol 13, 61–66 (2011). https://doi.org/10.1007/s12094-011-0618-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-011-0618-9