Abstract
Manganese peroxidase (MnP), a microbial ligninolytic enzyme which plays significant role in lignin and melanoidin degradation has gained much attention in the field of industry. In the present study, 15 ligninolytic bacteria were isolated from the soil sample of Similipal Biosphere Reserve (SBR) and screened for MnP activity. The most efficient MnP-producing bacterium HNB5 was evaluated for alkali lignin and maillard reaction products (MRPs) degradation and identified as Enterobacter wuhouensis using 16S rRNA sequencing. This bacterium exhibited the highest MnP activity of 2.6 U mL−1 min−1 in un-optimized conditions. Further, optimization using response surface methodology E. wuhouensis showed increased MnP activity of 4.11 U mL−1 min−1 at pH 6.3, temperature 37 °C, substrate concentration 1.05%, and time 144 h. In both FT-IR and UV–Vis spectrophotometry analyses of control and bacterium degraded MRPs, the reduction in Maillard product colour was correlated with shifting absorption peaks. Also, the GC–MS analysis data showing a change in functional group revealed the rise of novel peaks caused due to the degradation of MRPs complex. The phytotoxicity study was conducted for bacterial degraded MRPs medium revealed that toxicity of the medium decreased after bacterial treatment. The findings of the current study suggest that the manganese MnP produced by E. wuhouensis isolated from SBR soil sample may be employed for bioremediation purposes to degrade MRPs.
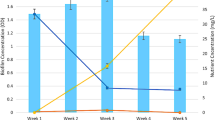
Graphical abstract










Similar content being viewed by others
Availability of data and materials
All data generated or analysed during this study are included in this article.
References
Chandra R, Kumar V (2017) Phytoextraction of heavy metals by potential native plants and their microscopic observation of root growing on stabilised distillery sludge as a prospective tool for in situ phytoremediation of industrial waste. Environ Sci Pollut Res 24:2605–2619
Tripathi S, Sharma P, Singh K, Purchase D, Chandra R (2021) Translocation of heavy metals in medicinally important herbal plants growing on complex organometallic sludge of sugarcane molasses-based distillery waste. Environ Technol Innov 22:101434
Satyawali Y, Balakrishnan M (2008) Wastewater treatment in molasses-based alcohol distilleries for COD and color removal: a review. J Environ Manage 86:481–497. https://doi.org/10.1016/j.jenvman.2006.12.024
Liang Z, Wang Y, Zhou Y, Liu H (2009) Coagulation removal of melanoidins from biologically treated molasses wastewater using ferric chloride. Chem Eng J 152:88–94. https://doi.org/10.1016/j.cej.2009.03.036
Liang Z, Wang Y, Zhou Y, Liu H, Wu Z (2009) Variables affecting melanoidins removal from molasses wastewater by coagulation/flocculation. Sep Purif Technol 68:382389. https://doi.org/10.1016/j.seppur.2009.06.011
Onyango MS, Kittinya J, Hadebe N, Ojijo VO, Ochieng A (2011) Sorption of melanoidin onto surfactant modified zeolite. Chem Ind Chem Eng Q 17:385–395
Chandra R, Ghosh A, Jain RK, Singh S (2006) Isolation and characterization of two potential pentachlorophenol degrading aerobic bacteria from pulp paper effluent sludge. J Gen Appl Microbiol 52:125–130
Dwyer J, Kavanagh L, Lant P (2008) The degradation of dissolved organic nitrogen associated with melanoidin using a UV/H2O2 AOP. Chemosphere 71:1745–1753. https://doi.org/10.1016/j.chemosphere.2007.11.027
Kim SB, Hayase F, Kato H (1985) Decolorization and degradation products of melanoidins on ozonolysis. Agric Biol Chem 49:785–792. https://doi.org/10.1080/00021369.1985.10866785
Saikia S, Yadav M, Hoque RA, Yadav HS (2022) Bioremediation mediated by manganese peroxidase—an overview. Biocatal Biotransform 1–13
Bhujbal SK, Ghosh P, Vijay VK, Rathour R, Kumar M, Singh L, Kapley A (2022) Biotechnological potential of rumen microbiota for sustainable bioconversion of lignocellulosic waste to biofuels and value-added products. Sci Total Environ 814:152773
Yadav S, Chandra R (2013) Detection of persistent organic compounds from biomethanated distillery spent wash (BMDS) and their degradation by manganese peroxidase and laccase producing bacterial strains. J Environ Biol 34:755. https://doi.org/10.1007/s11274-018-2416-9
Zhang C, Hu M, Di Maio F, Sprecher B, Yang X, Tukker A (2022) An overview of the waste hierarchy framework for analyzing the circularity in construction and demolition waste management in Europe. Sci Total Environ 803:149892
Kumar V, Chandra R (2018) Characterisation of manganese peroxidase and laccase producing bacteria capable for degradation of sucrose glutamic acid-Maillard reaction products at different nutritional and environmental conditions. World J Microbiol Biotechnol 34:32
Woo HL, Hazen TC, Simmons BA, DeAngelis KM (2014) Enzyme activities of aerobic lignocellulolytic bacteria isolated from wet tropical forest soils. Syst Appl Microbiol 37:60–67
Goyal A, Ghosh B, Eveleigh D (1991) Characteristics of fungal cellulases. Biores Technol 36:37–50
Raj A, Kumar S, Haq I, Singh SK (2014) Bioremediation and toxicity reduction in pulp and paper mill effluent by newly isolated ligninolytic Paenibacillus sp. Ecol Eng 71:355–362
Dutta S, Chatterjee S, Mukherjee I, Saha R, Singh BP (2017) Fabrication of ZnS hollow spheres and RGO-ZnS nanocomposite using cysteamine as novel sulfur source: photocatalytic performance on industrial dyes and effluent. Ind Eng Chem Res 56:4768–4778. https://doi.org/10.1021/acs.iecr.7b00107
Sarabia LA, Ortiz MC (2009) Response surface methodology. Compr Chemom 21:345–390. https://doi.org/10.1016/b978-044452701-1.00083-1
Misra RC, Sahoo HK, Pani DR, Bhandari DC (2013) Genetic resources of wild tuberous food plants traditionally used in Similipal Biosphere Reserve, Odisha, India. Genet Resour Crop Evol 60:2033–2054. https://doi.org/10.1007/s10722-013-9971-6
Pangallo D, Šimonovičová A, Chovanová K, Ferianc P (2007) Wooden art objects and the museum environment: identification and biodegradative characteristics of isolated microflora. Lett Appl Microbiol 45:87–94
Bholay AD, Borkhataria BV, Jadhav PU, Palekar KS, Dhalkari MV, Nalawade PM (2012) Bacterial Lignin peroxidase: a tool for biobleaching and biodegradation of industrial effluents. Univ J Environ Res Technol 1
Arora DS, Chander M, Gill PK (2002) Involvement of lignin peroxidase, manganese peroxidase and laccase in degradation and selective ligninolysis of wheat straw. Int Biodeterior Biodegradation 50:115–120
Coico R (2006) Gram staining. Curr Protoc Microbiol 1:A-3C
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics, 23(21):2947–2948
Garai D, Kumar V (2013) A Box–Behnken design approach for the production of xylanase by Aspergillus candidus under solid state fermentation and its application in saccharification of agro residues and Parthenium hysterophorus L. Ind Crops Prod 44:352–363
Smitha KV, Pradeep BV (2017) Application of Box-Behnken design for the optimization of culture conditions for novel fibrinolytic enzyme production by Bacillus altitudinis S-CSR 0020. J Pure Appl Microbiol 11(3):1447–1456
Mahapatra M, Karmakar M, Dutta A, Singha NR (2018) Fabrication of composite membranes for pervaporation of tetrahydrofuran-water: Optimization of intrinsic property by response surface methodology and studies on vulcanization mechanism by density functional theory. Korean J Chem Eng 35:1889–1910
Allen DC, Grethlein HE, Converse AO (1983) Process studies for enzymatic hydrolysis using high solids slurries of acid pretreated mixed hardwood. Biotechnol Bioeng Sympos 13:830567
Chang YC, Choi D, Takamizawa K, Kikuchi S (2014) Isolation of Bacillus sp. strains capable of decomposing alkali lignin and their application in combination with lactic acid bacteria for enhancing cellulase performance. Biores Technol 152:429–436
Bharagava RN, Chandra R, Rai V (2009) Isolation and characterization of aerobic bacteria capable of the degradation of synthetic and natural melanoidins from distillery effluent. World J Microbiol Biotechnol 25:737–744
Chandra R, Kumar V, Tripathi S (2018) Evaluation of molasses-melanoidin decolourisation by potential bacterial consortium discharged in distillery effluent. 3Biotech 8:187
Miyata N, Mori T, Iwahori K, Fujita M (2000) Microbial decolorization of melanoidin-containing wastewaters: combined use of activated sludge and the fungus Coriolus hirsutus. J Biosci Bioeng 89:145–150
Yadav S, Chandra R (2012) Biodegradation of organic compounds of molasses melanoidin (MM) from biomethanated distillery spent wash (BMDS) during the decolourisation by a potential bacterial consortium. Biodegradation 23:609–620
Shi Y, Chai L, Tang C, Yang Z, Zhang H, Chen R, Zheng Y (2013) Characterization and genomic analysis of kraft lignin biodegradation by the beta-proteobacterium Cupriavidus basilensis B-8. Biotechnol Biofuels 6:1–14
Chandra R, Yadav S, Kumar V (2015) Microbial degradation of lignocellulosic waste and its metabolic products. Environm Waste Manag 249–298
Zhang S, Xiao J, Wang G, Chen G (2020) Enzymatic hydrolysis of lignin by ligninolytic enzymes and analysis of the hydrolyzed lignin products. Biores Technol 304:122975
Haal A, Kazak U (1989) A broadband coaxial ridged horn antenna. In: 1989 19th European microwave conference, pp 247–252
Jaszek M, Żuchowski J, Dajczak E, Cimek K, M Gra̢z, K Grzywnowicz (2006) Ligninolytic enzymes can participate in a multiple response system to oxidative stress in white-rot basidiomycetes: Fomes fomentarius and Tyromyces pubescens. Int Biodeterior Biodegradation 58:168–175
Gu FL, Kim JM, Abbas S, Zhang XM, Xia SQ, Chen ZX (2010) Structure and antioxidant activity of high molecular weight Maillard reaction products from casein–glucose. Food Chem 120:505–511
González T, Terrón MC, Yagüe S, Zapico E, Galletti GC, González AE (2000) Pyrolysis/gas chromatography/mass spectrometry monitoring of fungal-biotreated distillery wastewater using Trametes sp. I-62 (CECT 20197). Rapid Commun Mass Spectrom 14:1417–1424
Chandra R, Bharagava RN, Rai V (2008) Melanoidins as major colourant in sugarcane molasses based distillery effluent and its degradation. Biores Technol 99:4648–4660
Yadav S, Chandra R, Rai V (2011) Characterization of potential MnP producing bacteria and its metabolic products during decolourisation of synthetic melanoidins due to biostimulatory effect of D-xylose at stationary phase. Process Biochem 46:1774–1784
Kumar V, Agrawal S, Shahi SK, Motghare A, Singh S, Ramamurthy PC (2022) Bioremediation potential of newly isolated Bacillus albus strain VKDS9 for decolourization and detoxification of biomethanated distillery effluent and its metabolites characterization for environmental sustainability. Environ Technol Innov 26:102260. https://doi.org/10.1016/j.eti.2021.102260
Kheti NK, Rath S, Thatoi H (2023) Screening and optimization of manganese peroxidase (MnP) production by Pseudoduganella violacea (SMB4), a bacterial isolate from Similipal Biosphere Reserve, Odisha and evaluation of Maillard reaction products degradation. Sustain Chem Environ 100009
Acknowledgements
Authors are grateful to the HOD of Department of Biotechnology, Maharaja Sriram Chandra Bhanja Deo University, Baripada, Odisha, India for providing infrastructure facilities to conduct the present work. The author(s) are grateful to AGL, IIT (ISM), Dhanbad for FTIR analyses, and visualization(s) used in this study. The author(s) are also grateful to Dr. Rajiv Chandra Dev Goswami, Research associate, Guwahati Biotech Park, Science Technology and Climate Change Department, Amingaon , Guwahati for providing technical support during GC-MS analysis used in this study.
Author information
Authors and Affiliations
Contributions
HT developed and supervised the entire research project and made over all editing in manuscript. In this work, SR and NK executed experiments and analyzed the data, as well as helped in writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thatoi, H., Rath, S. & Kheti, N.K. Optimisation of Manganese Peroxidase (MnP) Activity of Enterobacter wuhouensis Using Response Surface Method and Evaluation of Its Maillard Reaction Products Along with Lignin Degradation Ability. Indian J Microbiol 63, 604–620 (2023). https://doi.org/10.1007/s12088-023-01120-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-023-01120-6