Abstract
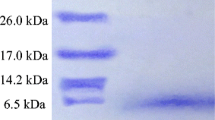
Enterococcus faecium FH 99 was isolated from human faeces and selected because of its broad spectrum of inhibitory activity against several Gram-positive foodborne spoilage and pathogenic bacteria. Ent. faecium FH 99 accumulates enterocin in large number in early stationary phase of the growth. The enterocin FH 99 was stable over a wide pH range (2–10) and recovered activity even after treatment at high temperatures (10 min at 100°C). The enterocin was subjected to different purification techniques viz., gel filteration, cation exchange chromatography and reverse-phase high-performance liquid chromatography. The activity was eluted as one individual active fraction. SDSPAGE revealed a molecular weight of less than 6.5 kDa. Studies carried out to identify the genetic determinants for bacteriocin production showed that this trait may be plasmid encoded as loss in both of the plasmids (size>chromosomal DNA) led to loss in bacteriocin production by Ent. faecium FH 99. Ent. faecium strain FH 99 is a newly discovered high bacteriocin producer with Activity Units 1.8 × 105 AU ml−1 and its characteristics indicate that it may have strong potential for application as a protective agent against pathogens and spoilage bacteria in foods.
Similar content being viewed by others
References
Jack RW, Tagg JR and Ray B (1995) Bacteriocins of gram positive bacteria. Microbiol Rev 59:171–200
Klaenhammer TR (1993) Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 12:39–86
Nes IF, Diep DB, Havarstein LS, Brurberg MB, Eijsink V and Holo H (1996) Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leewenhoek 70:113–128
Franz CMAP, Holzapfel WH and Stiles ME (1999) Enterococci at the crossroads of food safety? Int J Food Microbiol 47:1–24
Centeno JA, Menendez S, Hermida M and Rodriguez-Otero JL (1999) Effects of the addition of Enterococcus faecalis in Cebreiro cheese manufacture. Int J Food Microbiol 48: 97–111
Saraswat DS, Clark WS and Reinbold GM (1963) Selection of medium for the isolation and enumeration of enterococci in dairy products. J Milk Food Technol 26:114–117
Uhlman U, Schillinger U, Rupnow JR and Holzapfel WH (1992) Identification and characterization of two bacteriocin-producing strains of Lactococcus lactis isolated from vegetables. Int J Food Microbiol 16:141–151
De Man JC, Rogosa M and Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Appl Bacteriol 23:130–135
Pospiech A and Neumann B (1995) A versatile quick-prep of genomic DNA from Gram-positive bacteria. Trends in Genetics 11:217–218
Cheng S, McCleskey FK, Gress MJ, Petroziello JM, Liu R, Namdari H, Beninga K, Salmen A and Del Vecchio VG (1997) A PCR assay foe identification of Enterococcus faecium. J Clin Microbiol 35:1248–1250
Barefoot SF and Klaenhammer TR (1983) Detection and activity of Lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol 45:1808–1815
Biswas SR, Ray P, Johnson MC and Ray B (1991) Influence of growth conditions on the production of a bacteriocin AcH by Pediococcus acidilactici H. Appl Environ Microbiol 57: 1265–1267
Gupta H and Malik RK (2007) Incidence of virulence in bacteriocin producing enterococcal isolates. Lait 87:587–601
Lowry OH, Rosebrough NJ, Farr AL and Randall RJ (1951) Protein measurement with the Folin Phenol reagent. J Biol Chem 1993:265–275
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
O’sullivan DJ and Klaehammer TR (1993) Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl Environ Microbiol 59: 2730–2733
Giraffa G, Carminati D and Neviani E (1997) Enterococci isolated from dairy products: a review of risks and potential technological use. J Food Prot 60:732–738
Hugas M, Garriga M and Aymerich MT (2003) Functionality of enterococci in meat products. Int J Food Microbiol 88: 223–233
Mikes Z, Ferencik M, Janhova E, Erbringer L and Ciznar I (1995), Hypocholesterolemic and immunostimulatory effects of orally applied Enterococcus faecium M-74 in man. Folia Microbiologica 40:639–646
Folquie Moreno MR, Sarantinopoulos P, Tsakalidou E and De Vuyst L (2006) The role and application of enterococci in food and health. Int J Food Microbiol 106:1–24
Achemchem F, Martinez-Bueno M, Abrini J, Valdivia E and Maqueda M (2005) Enterococcus faecium F58, a bacteriocinogenic strain naturally occurring in Jben, a soft, farmhouse goat’s cheese made in Morocco. J Appl Microbiol 99: 141–150
Giraffa G, Neviani E and Torri Tarelli G (1994) Antilisterial activity by enterococci in a model predicting the temperature evolution of Taleggio, an Italian Soft Cheese. J Dairy Sci 77: 1176–1182
Du Toit M, Franz CMAP, Dicks LMT and Holzapfel H (2000) Preliminary characterization of bacteriocins produced by Enterococcus faecium and Enterococcus faecalis isolated from pig faeces, J Appl Microbiol 88:482–494
Simonova M and Laukova A (2004) Isolation of faecal Enterococcus faecium strains from rabbits and their sensitivity to antibiotics and ability to bacteriocin production. Bull Vet Institute Pulawy 48:383–386
Kang JH and Lee MS (2005) Characterization of a bacteriocin produced by Enterococcus faecium GM-1 isolated from an infant. J Appl Microbiol 98:1169–1176
Franz CMAP, Stiles ME, Schleifer KH and Holzapfel WH (2003) Enterococci in foods — a conundrum for food safety. Int J Food Microbiol 88:105–122
Galvez A, Gimenez-Gallego G, Maqueda M and Valdivia E (1989) Purification and amino acid composition of peptide antibiotic AS-48 produced by Streptococcus (Enterococcus) faecalis subsp. liquefaciens S-48. Antimicrob Agents Chemother 33:437–441
Tomita H, Fujimoto S, Tanimoto K and Ike Y (1997) Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pPD1. J Bacteriol 179:7843–7855
Sparo MD, Castro MS, Andino PJ, Lavigne MV, Ceriani C, Gutierrez GL, Fernandez MM, De Marzi MC, Malchiodi EL and Manghi MA (2006) Partial characterization of enterocin MR99 from corn silage isolate of Enterococcus faecalis. J Appl Microbiol 100:23–34
Simonetta AC, Moragues de Velasco LG and Frison LN (1997) Antibacterial activity of enterococci strains against Vibrio cholerae. Lett Appl Microbiol 24:139–143
Hardwood VJ, Delahoya NC, Ulrich RM, Kramer MF, Whitlock JE, Garey JR and Lim DV (2004) Molecular confirmation of Enterococcus faecalis and E. faecium from clinical, faecal and environmental sources. Lett Appl Microbiol 38: 476–482
Keppler K, Geisen R and Holzapfel WH (1994) An -amylase sensitive bacteriocin of Leuconostoc carnosum. Food Microbiol 11:39–45
Folquie Moreno MR, Callewaert R, Devriese B, Beeumen JV and De Vuyst L (2003) Isolation and biochemical characterization of enterocins produced by enterococci from different sources. J Appl Microbiol 94:214–229
Abriouel H, Lucas R, Ben Omar N, Valdivia E, Maqueda M, Martinez-Canamero M and Galvez A (2005) Enterocin AS-48 RJ: a variant of enterocin AS-48 chromosomally encoded by Enterococus faecium RJ16 isolated from food. Sys Appl Microbiol 28:383–397
Herranz C, Casaus P, Mukhopadhyay S, Martinez JM, Rodriguez JM, Nes IF, Hernandes PE and Cintas LM (2001) Enterococcus faecium P21: a strain occurring naturally in dry fermented sausages producing the class II bacteriocins enterocin A and enterocin B. Food Microbiol 18:115–131
Hechard Y, Berjeaud JM and Cenatiempo Y (1999) Characterization of the mesB gene and expression of bacteriocins by Leuconostoc mesnteroides Y105. Curr Microbiol 39: 265–269
Ganzle MG, Weber S and Hammes WP (1999) Effect of ecological factor on the inhibitory spectrum and activity of bacteriocins. Int J Food Microbiol 46:1637–1645
Sabia C, Messi P, de-Niederhausern S, Manicardi G and Bondi M (2004) Study of two bacteriocins produced by Enterococcus casseliflavus and Enterococcus faecalis. Lett Appl Microbiol 38:99–105
Ohmomo S, Murata S, Katayama N, Nitisinprasart S, Kobayashi M, Nakajima T, Yajima M and Nakanishi K (2000) Purification and some characteristics of enterocin ON-157, a bacteriocin produced by Enterococcus faecium NIAI 157. J Appl Microbiol 88:81–89
De Vuyst L, Moreno MRF and Revets H (2003) Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins. Int J Food Microbiol 84:299–318
Terzaghi BE and Sandine WE (1975) Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol 29:807–813
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, H., Malik, R.K., Bhardwaj, A. et al. Purification and characterization of enterocin FH 99 produced by a faecal isolate Enterococcus faecium FH 99. Indian J Microbiol 50, 145–155 (2010). https://doi.org/10.1007/s12088-010-0039-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-010-0039-4