Abstract
Introduction
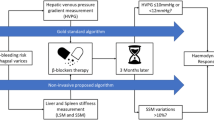
Non-selective beta-blockers (NSBB) are used for primary prophylaxis in patients with liver cirrhosis and high-risk varices (HRVs). Assessing therapeutic response is challenging due to the invasive nature of hepatic venous pressure gradient (HVPG) measurement. This study aims to define a noninvasive machine-learning based approach to determine response to NSBB in patients with liver cirrhosis and HRVs.
Methods
We conducted a prospective study on a cohort of cirrhotic patients with documented HRVs receiving NSBB treatment. Patients were followed-up with clinical and elastography appointments at 3, 6, and 12 months after NSBB treatment initiation. NSBB response was defined as stationary or downstaging variceal grading at the 12-month esophagogastroduodenoscopy (EGD). In contrast, non-response was defined as upstaging variceal grading at the 12-month EGD or at least one variceal hemorrhage episode during the 12-month follow-up. We chose cut-off values for univariate and multivariate model with 100% specificity.
Results
According to least absolute shrinkage and selection operator (LASSO) regression, spleen stiffness (SS) and liver stiffness (LS) percentual decrease, along with changes in heart rate (HR) at 3 months were the most significant predictors of NSBB response. A decrease > 11.5% in SS, > 16.8% in LS, and > 25.3% in HR was associated with better prediction of clinical response to NSBB. SS percentual decrease showed the highest accuracy (86.4%) with high sensitivity (78.8%) when compared to LS and HR. The multivariate model incorporating SS, LS, and HR showed the highest discrimination and calibration metrics (AUROC = 0.96), with the optimal cut-off of 0.90 (sensitivity 94.2%, specificity 100%, PPV 95.7%, NPV 100%, accuracy 97.5%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Portal hypertension (PH) is a significant complication in patients with chronic advanced liver disease, leading to the development of esophageal varices (EVs), ascites, hepatic encephalopathy (HE), and hepatorenal syndrome (HRS), which contribute to increased morbidity and mortality [1]. The gold-standard method for diagnosing clinically significant portal hypertension (CSPH) is hepatic venous pressure gradient (HVPG), an invasive and expensive procedure available only in specialized centers [2]. Therefore, non-invasive methods have been investigated to serve as surrogates of HVPG measurement to stage PH [3,4,5]. Despite the development and validation of several laboratory values-based scores, liver stiffness (LS) has emerged as a non-invasive, reliable, and widely accepted method for assessing the degree of liver fibrosis, cirrhosis, and stratification of PH, thus serving as a non-invasive marker for liver disease management and risk-stratification [3]. LS has been widely adopted and validated as an EVs screening surrogate, with the Baveno VI guidelines confidently recommending that compensated cirrhotic patients with LS < 20 kPa and platelets > 150,000/mm3 had a 95% possibility for not developing high-risk varices (HRVs) [6]. During the transition period between previous and current guidelines, spleen stiffness (SS) has emerged as a comparable technique, if not superior, to LS in staging and risk prediction in patients with CSPH [7, 8]. In fact, according to Baveno VII recommendations, elastography is sufficiently accurate to identify CSPH in clinical practice [9]: in non-obese patients with compensated advanced chronic liver disease (cACLD), LS ≥ 25 kPa is sufficient to rule-in CSPH (specificity and positive predictive value > 90%). In addition, SS cutoff has been introduced to rule-in CSPH for viral-related cirrhosis (SS ≥ 50 kPa) and to identify those patients at low probability of HRVs that are required to undergo EGD according to Baveno VI criteria, in whom endoscopy can be avoided (SS ≤ 40 kPa) [9]. Implementing an elastography-driven definition of CSPH has also had significant therapeutic implications, particularly regarding indications for non-selective beta-blocker (NSBB) therapy. According to the new Baveno VII statements, the decision to treat with NSBB should be independent of HVPG measurements, and patients currently on NSBB are not required to undergo screening EGD for the detection of EVs [9]. However, it is still unclear how to monitor NSBB hemodynamic response without HVPG measurements, especially considering that the number needed to treat (NNT) in primary prophylaxis ranges between 5 and 13 [10]. The non-invasive strategy to screen and treat patients with CSPH carries the risk of being unable to monitor the efficacy of NSBB fully, thus exposing non-responders to an increased risk of variceal hemorrhage.
There are few studies that explored the role of liver and spleen elastography on the correlation between SS or LS values with HVPG measurements at baseline and in response to NSBB treatment [11,12,13,14]. According to two studies, SS appears to be the best predictor of hemodynamic response to NSBB [13, 14], and a percentual decrease of at least 10% from individual values is highly predictive of response [13]. On the contrary, Binzberger et al. [12] demonstrated that neither LS nor SS could reliably predict response to NSBB. These preliminary findings need to be further validated and explored in other clinical settings, in order to define if elastography can be used to monitor response to NSBB.
Thus this study aims to create a machine-learning algorithm to predict non-invasively response to NSBB in patients with HRVs on primary prophylaxis for variceal hemorrhage who were undergoing serial measurements of LS and SS in the first 12 months following NSBB administration.
Materials and methods
Study design, patient follow-up, and data collection
The present study is a prospective observational cohort study conducted at a single center (Trieste University Hospital) enrolling patients referred to the Liver Clinic Unit. The study consisted of two parts: the first part (1st May 2018 to 31st December 2020) aimed to derive a prediction model, whereas the second part (1st January 2021 to 31st December 2021) was designed to enroll a cohort of patients where the model could be validated. We enrolled consecutive patients with a diagnosis of liver cirrhosis and the presence of HRVs and an indication for primary prophylaxis with NSBB for first variceal bleeding prevention. The diagnosis of liver cirrhosis was established utilizing a combination of clinical, biochemical, and ultrasound imaging (e.g., nodular liver surface, coarse liver echotexture), and/or histological examination [15, 16]. The prescription and initiation of NSBB therapy were part of the patient's routine therapeutic course, and their participation in the study did not interfere with the established practice of treatment, which was assessed following current guidelines.
Each eligible patient was evaluated at baseline through clinical assessment (physical examination, vital parameters such as heart rate and arterial blood pressure, and anthropometric characteristics such as height, weight, and calculation of body mass index) and with the following laboratory tests: white blood cell count (WBC), hematocrit, hemoglobin, platelet count, INR, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), sodium, potassium, total bilirubin, albumin, and creatinine. The Child–Pugh [17] and model for end-stage liver disease (MELD) [18] scores were calculated for each patient. On the same day, each patient underwent liver and spleen elastography measurements and evaluation of ultrasonographic parameters such as portal vein diameter, portal flow velocity, spleen bipolar diameter, and spleen surface measured at the hilum. After this initial screening, patients with LS > 20 kPa and platelet count < 150.000 × 109 cells/L underwent EGD for EVs screening within 10 days. Patients with endoscopic evidence of HRVs and without NSBB contraindications were prescribed an NSBB with dose titration as suggested by the American Association of the Study of the Liver guidelines [1]. Propranolol was started with a dose of 20–40 mg orally twice a day, with dose adjusting every 2–3 days (maximum daily doses of 320 mg/day in patients without ascites and 160 mg/day in patients with ascites). Carvedilol was started with a dose of 6.25 mg once a day and a dose adjustment every 3 days (maximum daily dose of 12.5 mg/day). Each patient who started NSBB therapy was closely monitored in the first 3 weeks for dose titration and then re-evaluated after 3 months (physical examination, liver, and spleen elastography, and laboratory exams), six (physical examination, liver, and spleen elastography, liver ultrasound examination, and laboratory exams), and twelve (physical examination, liver, and spleen elastography, liver ultrasound examination, laboratory exams, and EGD) months after therapy initiation, as shown in Fig. 1.
Description of follow-up timeline from enrollment. Eligible patients underwent baseline clinical assessment, laboratory tests, and elastography measurements liver stiffness > 20 kPa and platelet count < 150.000 × 109 cells/L underwent EGD for EVs screening within 10 days. Patients with HRVs were prescribed NSBBs, and re-evaluated at 3/6 months, and 1-year post-therapy initiation. a Flowchart reporting patients who completed follow-up in the derivation cohort, while b reports patients who completed follow-up in the validation cohort
Inclusion criteria
The inclusion criteria for the study were: age > 18 years with liver cirrhosis regardless of etiology, who had undergone esophagogastroduodenoscopy (EGD) according to Baveno VI criteria (LS > 20 kPa and platelet count < 150.000 × 109 cells/L) [19] with evidence of HRVs and candidate to NSBB therapy.
Exclusion criteria
We categorically excluded patients with previously diagnosed and/or treated for hepatocellular carcinoma (HCC), portal vein thrombosis, previous treatment with NSBB or endoscopic variceal band ligation (EBL), with contraindications to NSBB administration (heart rate < 50 bpm, systolic blood pressure < 100 mmHg, aortic disease, atrioventricular blocks, severe peripheral angiopathy, asthma or chronic obstructive pulmonary disease of any severity), pregnant female patients, severe obesity (BMI > 40), and known hematological disease of any kind. Furthermore, considering that ongoing liver injury, as delineated by guidelines, typically influences subsequent endoscopic evaluations for patients with suspected clinically significant portal hypertension (CSPH) [1] and may impact NSBB response prediction, we chose to exclude all patients with ongoing liver injury (e.g., ongoing alcohol abuse, untreated HCV or HBV infection, or autoimmune flares without immunosuppressive treatment). In addition, due to limited resources, we made the decision to exclude from the final analysis any patients who experienced variceal hemorrhage prior to their initial elastography follow-up after beginning NSBB treatment (i.e., 3 months after first administration).
Endoscopic assessment of esophageal varices
EGD was performed by the staff of the Gastrointestinal and Endoscopy Service at Trieste University Hospital using PENTAX endoscopic devices EPK-i7010 series. All the instruments are at high definition. The exploration of the esophagus was performed spending at least 3 min initially in a deflated state, obtaining at least three images (upper, middle, and lower esophagus), and then at maximum insufflation using CO2, again obtaining at least three images (upper, middle, and lower esophagus). The images obtained were stored in the EndoxWeb software available in the hospital management system. The endoscopic classification of EVs was performed according to the Beppu Classification [20]. HRVs were defined as EVs ≥ F2 with/without red signs or F1 with red signs. For each EGD, EVs classification was assessed by a second experienced endoscopist, blind to the first endoscopist grading, using the stored image obtained by the endoscopist who performed the EGD. When agreement was not reached between the two classifiers, a third endoscopist was consulted to confirm one of the two classifications. All the endoscopists are experts in EVs diagnostic and therapeutic evaluation both in elective and urgent settings.
Outcome definition
We defined a binary outcome of response to NSBB according to endoscopic evaluation or the presence of variceal hemorrhage. We defined as “responders” the patients with stationary or downstaged variceal grading at the 12-month EGD. In contrast, we defined as “non-responders” the patients with upstaging variceal grading at the 12-month EGD or at least one variceal hemorrhage episode during the 12-month follow-up.
Elastography measurement
Liver and Spleen Stiffness were measured using a Philips Affiniti 70 (ElastPQ Protocol) ultrasonography system with a 1–5 MHz convex probe [21,22,23,24,25,26]. All measurements were performed by four experienced operators (> 500 elastography examinations each). Patients were positioned in supine decubitus with the right arm (liver) or left arm (spleen) in maximal abduction to increase the intercostal acoustic window. The region of interest (ROI) was placed between the VII and VIII segments at least 1.5 cm from the hepatic capsule (LS) and at the splenic hilum or lower pole at least 1 cm from the splenic capsule (SS) [22]. The ROI was accurately located in an area without large liver vessels, bile ducts, and rib shadows. During the acquisition, the patient was requested to hold his/her breath for 5 s [27]. All measures obtained after a deep inspiration, maximal expirations, and Valsalva maneuver were discarded. In some cases, breath-hold was practiced with the patient prior to initiating elastography. Ten different valid elastography measurements were obtained in all subjects, both in the liver and the spleen, and the median value was used. The measure obtained was acquired only if its standard deviation was < 30% [27]. We defined “technical failure” as the impossibility of obtaining any value or an IQR/M ≥ 0.30 and selected values with an IQR/M < 0.30 [27]. All patients were examined after overnight fasting and without caffeine intake in the previous 3 h. Each physician performing elastography examination was blind to the initial endoscopic patient status and was not informed about the initial endoscopic status or development of complications (e.g., variceal hemorrhage) in any of the follow-up appointments.
Adaptation of Kim et al.’s model
Kim et al. [14] proposed a univariate model based on SS. In particular, the linear predictor (LP) was calculated as follows: 0.0490–2.8345 × ΔSS, where ΔSS was defined as SS (3rd-Month-Follow-up) – SS(Enrollment). In addition, Kim et al. employed the Siemens Acuson S2000TM ultrasound system (Siemens AG, Erlangen, Germany) to perform LS and SS measurements and provided results in m/s. Given that our system provided measurements in kPa, they were converted into m/s using the following conversion: Measurem/s = √(MeasurekPa/3).
Statistical analysis
According to our sample size, the Shapiro–Wilk test was performed to verify the normal distribution of variables [28], whose results indicated the absence of normally distributed variables, which were therefore reported as median (Quartile 1; Quartile 3). Differences between continuous variables were examined using the Mann–Whitney U test. Variable correlations were analyzed using the Spearman’s rank correlation test [29]. Exploratory data analysis in the derivation cohort revealed moderate to high correlations between variables, thus the need for variable shrinkage to avoid overfitting. Therefore, we employed the least absolute shrinkage and selection operator (LASSO) logistic regression [30] by selecting a penalization factor (i.e., lambda, λ) across tenfold cross-validation with the lowest cross-validation error, which resulted in a λ = 6.70. Then, we applied the penalization factor and selected variables with an absolute value of the β coefficient >|0.01|, which resulted in the selection of three variables: 3-month LS percentual decrease, 3-month SS percentual decrease, and 3-month HR percentual decrease. Univariate logistic regression was performed for each of the three variables with tenfold cross-validation, followed by multivariate logistic regression using all three variables. For each model, the primary performance measure was measured using the area under the receiver-operating characteristic curve (AUROC), and calibration with Nagelkerke Pseudo-R2, Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC). Statistical comparison of the AUROC for the separate models was performed using DeLong’s Test. The model was developed to predict response to NSBB. Therefore, for cut-off analysis, it is crucial to identify those patients who do not respond to NSBB treatment. Achieving a high specificity is important for this group, because false positives must be very rare so that patients who do not respond to treatment will be closely monitored or sent to more invasive diagnostic follow-up tests. Therefore, we planned to use a cut-off value that achieved a specificity of 100% (or closest to 100% if none reached 100%) and to compare sensitivities using McNemar’s matched pairs test. Each derived model and cut-off values were then evaluated in the validation cohort by AUROC analysis. For all analyses, two-sided statistical significance was defined as p < 0.05 [22]. The model provided in our cohort of patients was compared to the model by Kim et al. [14] in terms of AUROC (DeLong’s Test), AIC, BIC, and Nagelkerke Pseudo-R2. Data were analyzed using Python (Version 3.11.2) using numpy, matplotlib, pandas, scipy and sklearn packages.
Results
A total of 165 patients were identified as eligible to be enrolled in the derivation cohort, however, six patients were excluded for the presence of contraindications to NSBB, five patients were excluded for concurrent diagnosis of HCC, two patients were excluded for the detection of portal vein thrombosis, and two did not agree to participate in the study. Therefore, a total of 150 patients were enrolled in the derivation cohort (Fig. 1). Among these, twenty patients withdrew from the study due to NSBB adverse reactions, ten patients were lost to follow-up, and one patient died for unrelated causes, with 119 patients having completed the 12-month follow-up and being included in the study. For the validation cohort, 50 patients were identified as eligible. However, eight patients were excluded for contraindications to NSBB, and two patients were excluded for concomitant diagnosis of HCC. Therefore, a total of 40 patients were enrolled in the validation cohort (Fig. 1). Among these, two patients withdrew from the study due to NSBB adverse reactions, and four patients were lost to follow-up, with 34 patients having completed the 12-month follow-up and being included in the study. Patients' baseline characteristics and time-related changes of significant variables are reported in Tables 1 and 2, respectively.
In the derivation cohort group of non-responders (N = 43), six (13.95%) patients presented to the emergency department with variceal hemorrhage during the follow-up period, whereas the remaining 37 showed endoscopic upstaging of EVs grading. In the validation cohort group of non-responders (N = 12), two (16.66%) patients presented to the emergency department with variceal hemorrhage during the follow-up period, whereas the remaining ten showed endoscopic upstaging of EVs grading. In the derivation cohort of responders (N = 76), 56 (73.7%) patients showed downstaged EVs grading at the 12-month EGD, whereas 20 (26.3%) of patients showed stationary EVs grading at the 12-month EGD. In the validation cohort group of responders (N = 22), 13 (59.1%) patients showed downstaged EVs grading at the 12-month EGD, whereas 9 (40.9%) of patients showed stationary EVs grading at the 12-month EGD.
As reported in Table 2, median LS, HR, and SS values did not differ at enrollment evaluation between the responders and non-responders, their percentual decrease is statistically different across all follow-up appointments in both the derivation and validation cohort.
Model analysis
According to the statistical analysis description reported in the methods section, model metrics are summarized in Table 3 for univariate models derived from our cohort of patients (i.e., percentual decrease in value after 3 months of NSBB therapy for SS, LS, and HR), univariate model adapted from Kim et al. [14] formula, and the combined model derived from our cohort of patients that incorporated the three variables of interest (i.e., SS, LS, and HR).
As reported in Table 3, Model 4 showed statistically significant highest AUROC in both the training (0.96) and validation cohort (0.91), highest Nagelkerke Pseudo-R2 (0.70) and lowest AIC (79.3) and BIC (90.4) if compared to the univariate models derived from our cohort of patients and the univariate model derived from Kim et al. [14] formula.
For clarification purposes, to calculate the NSBB response probability using the combined model (i.e., Model 4), the following steps should be followed:
(i) obtain the percentual decrease of the variables at 3 months compared to index evaluation, and calculate the Linear Predictor (LP):
(ii) Once the LP is obtained, the probability of being a responder can be calculated using the following formula:
Model 4 is represented in a 3D graph in Fig. 2a, and the calibration plot for the derivation cohort (expected vs. observed risk) is illustrated in Fig. 2b.
a Presents a 3D visualization of a logistic regression model, depicting the relationship between the predicted outcome and three input variables, offering a comprehensive view of the model’s decision boundary. b Displays the calibration plot comparing the expected versus observed risk for the model, evaluating how well the predicted probabilities align with actual outcomes
Cut-off analysis and online calculator
Cut-off values, chosen to maximize specificity, are reported in Table 4, along with their sensitivity, NPV, PPV, and accuracy. For clarification purposes, we converted the univariate model cut-offs into the respective variable percentual change at 3 months. In the training cohort, the cut-off chosen for the combined model was 0.90, which showed a statistically significant higher sensitivity (94.2%) if compared to SS (78.8%, p = 0.022), LS (23.1%, p < 0.001), and HR (22.1%, p < 0.001). The application of the same cut-off value in the validation cohort resulted in similar findings with a specificity of 97%, and a statistically significant higher sensitivity (93.8%) if compared to SS (76.5%, p = 0.018), LS (31.2%, p < 0.001), and HR (21.5%, p < 0.001). To make the tool available for clinicians, we have developed an online app (http://esophagealvarices.org) that allows for point-of-care entry of the variables of interest and automatic generation of the predicted probability according to patients data.
Discussion
The primary aim of this study was to assess the feasibility of using LS and SS changes to determine the therapeutic response to treatment with NSBB in patients with liver cirrhosis and documented presence of HRVs. The main results of this study indicate that across the initial included variables, only three showed a stronger signal in the outcome prediction. Those three variables were the percentage changes at the third month of follow-up evaluation for SS, LS, and HR and that, as shown in Fig. 3, according to their univariate analysis, a decrease > 11.5% for SS, > 16.8% for LS, and > 25.3% for HR were selected to have a specificity of 100%, in order to provide lower risks of detecting false positives (i.e., those patients who have not responded to NSBB, that were predicted as responders by the model). In terms of cut-off metrics in the univariate models, the SS percentual decrease showed a statistically significant higher sensitivity (78.8%), if compared to LS (31.2%, p < 0.001) and HR (21.5%, p < 0.001) in both the training and the validation set. Besides, the SS univariate model showed the highest discriminative ability (AUROC = 0.89) if compared to LS (AUROC = 0.78, DeLong’s Test—p = 0.020) or HR (AUROC = 0.72, DeLong’s Test—p < 0.001) and it showed the best calibration metrics (AIC = 89.3, BIC 94.8). The application of the model provided by Kim et al., showed no statistically significant differences in AUROC with SS (AUROC = 0.88 vs. AUROC = 0.89 respectively). At the same time, it showed, similarly to our univariate SS-based model, statistically significant difference in AUROC with LS and HR.
After initial evaluation and detection of HRVs, patients should be treated with NSBB if they present no contraindications. According to our findings, before NSBB therapy initiation LS, SS, and HR should be registered and repeated after 3 months to determine NSBB response non-invasively. Responses can be either evaluated by considering the multivariate model (1) or by considering each variable singularly (2)
The multivariate model that incorporated SS, LS, and HR showed the highest discrimination in both the training (AUROC = 0.96) and validation (AUROC = 0.91), associated with the most performing calibration metrics (AIC = 79.3, BIC 90.4). We selected the cutoff value of 0.90, to maximize specificity up to 100%, which resulted also in a statistically significant higher sensitivity (94.2%), when compared to its univariate counterparts. The application of the model provided by Kim et al., showed no statistically significant differences in AUROC with our multivariate model (AUROC = 0.88 vs. AUROC = 0.96 respectively, DeLong’s Test – p = 0.037).
Our results on SS reflect those found by Marasco et al. [13], who reported that changes in SS were linearly related to changes in HVPG (r = 0.784, p value < 0.0001) and that changes in SS showed excellent discriminatory abilities (AUROC = 0.973), selecting a percentual decrease of -10% as the best cut-off to identify non-responders (sensitivity 100%, specificity 60%, NPV 100% and PPV 90%). In our study, the best SS cut-off to discriminate between responders and non-responders was a -11.5% at 3 months from the initial values. The authors also report that LS did not correlate with changes in HVPG (r = 0.107, p value = 0.655), similar to what has been reported by Binzberger et al.[12]. In our study, however, the univariate analysis of LS percentual changes resulted in a model with a less discriminative ability and calibration if compared to SS, but still resulted in one of the three most critical features according to LASSO regression to predict NSBB response. Rediberger et al. [31] reported that the linear correlation between LS and HVPG measurement became stronger in patients under treatment with NSBB, which was higher in responders (r = 0.864) than in non-responders (r = 0.535). Therefore, regarding LS, the study by Marasco et al. [13] may not have found sufficient correlation due to the limited sample size and that LS is a significantly less valid predictor for NSBB response if considered univariately.
Kim et al. [14] developed a univariate model in a training cohort of 106 patients and validated it in an external validation cohort of 63 patients. Response to NSBB was defined as a decrease in HVPG values ≥ 20% of the baseline value or an absolute value of HVPG < 12 mmHg after dose titration. The timing between HVPG measurements was not standardized amongst all patients, and the authors did not provide any information about the calibration metrics of the model. Their model showed excellent discrimination metrics in the training (AUROC = 0.801) and the validation cohort (AUROC = 0.848). As mentioned above, the application of the model proposed by Kim et al. [14] on our cohort of patients and our univariate model based on SS showed comparable calibration metrics, slightly in favor of our approach, thus highlighting how a percentual decrease if compared to an absolute value change may slightly better reflects changes in splenoportal hemodynamic.
However, both the McNemar that the Delong test did not provide statistically significant differences between our univariate SS model and the one that Kim et al. proposed [14] regarding discrimination and cutoff sensitivity analysis. The remarkable similarity to our findings, on the one hand, enables us to serve as an external validation group for a study involving a patient cohort with diverse geographic backgrounds (Korean vs. Italian patients). In addition, this has allowed us to surmount the most substantial limitation of the study, specifically the lack of HVPG measurement at our center. Consequently, this provided enhanced rigor to our results and the methodologies employed in determining the clinical response to NSBB therapy.
The true novelty of the current study lies in developing a combined model that considers both splenic/hepatic elastography values and changes in heart rate (i.e., three known different non-invasive indicators of NSBB response), and that the combined model showed the highest AUROCs in both the derivation and validation cohorts and to readily understand how the probability of NSBB changes across variations of each of the three variables.
The multivariate model exhibits the most efficient metrics in both discrimination and calibration. As shown in Fig. 2b, it is noteworthy that the expected versus observed risk is nearly entirely superimposable on the reference line. From a statistical point of view, according to LASSO regression, the highest weight of the model was given by SS values (2.68-fold higher than LS and 21.5-fold higher than HR), thus implying that most of the estimates are determined by SS and that LS and HR changes play only a minor role in the outcome prediction, thus providing insight on the relative weight of NSBB-induced effects on each of these non-invasive methods widely used to define CSPH and eventually monitor NSBB response. Also, the relevance of the model lies in the fact that there is a general paucity of data on assessing the hemodynamic response to NSBBs for HRVs. Furthermore, the prophylaxis of the first bleeding event is associated with a number needed to treat of 5–13 patients, which means that many patients are undergoing therapy without an actual efficacy on the primary prevention of bleeding from HRVs, thereby exposing them to potential side effects. These results have been highlighted by a meta-analysis by Zacharias et al. [32], which evaluated carvedilol compared to other NSBBs without finding any difference in efficacy on primary prophylaxis or the onset of adverse events. However, significantly more deaths, episodes of upper gastrointestinal bleeding, and serious adverse events occurred in the long-term trials. Therefore, it is essential to continue NSBB treatment only for patients who achieve a hemodynamic response; currently, HVPG measurement is the sole method for evaluating the efficacy of NSBB treatment.
Our study is limited by the unavailability of HVPG measurements at our center, and the absence of an external validation cohort from a separate center. However, our cohort is the largest to date studying NSBB response with elastography with consistent follow-up, resulting in high-quality data collection for the duration of the study. We did have a temporally separated validation cohort where we documented similar results from Kim et al. [14], which suggests that our findings are externally applicable.
Conclusions
Our study validates the result of Kim et al. [14] and defines a critical percentual decrease of SS in line with the findings of Marasco et al. [13], thus highlighting the potential role of spleen elastography in the prediction of NSBB response and as a practical ad non-invasive tool to monitor NSBB efficacy. Besides, the SS, LS, and HR combination provides a model with better prediction metrics than SS alone. Further studies are needed to validate our results and to verify if this technique can also be useful during the follow-up for monitoring portal pressure variations during the treatment and to possibly increase prediction capabilities of SS, LS, HR changes by reducing the re-evaluation intervals to the first 3 months after treatment initiation.
References
Garcia-Tsao G, Abraldes JG, Berzigotti A. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335.
Ferral H, Garcia-Pagàn JC, Schepis F. HVPG as a gold standard: consensus statements of panel 1. Portal hypertension VII. Springer International Publishing: Cham; 2022. p. 61–4 .https://doi.org/10.1007/978-3-031-08552-9_6
Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67:399–411.
Ravaioli F, Montagnani M, Lisotti A. Noninvasive assessment of portal hypertension in advanced chronic liver disease: an update. Gastroenterol Res Pract. 2018;2018:1–11.
Dajti E, Alemanni LV, Marasco G. Approaches to the diagnosis of portal hypertension: non-invasive or invasive tests? Hepat Med. 2021;13:25–36.
Franchis de R. Expanding consensus in portal hypertension. J Hepatol. 2015;63:743–752.
Hu X, Huang X, Hou J. Diagnostic accuracy of spleen stiffness to evaluate portal hypertension and esophageal varices in chronic liver disease: a systematic review and meta-analysis. Eur Radiol. 2021;31:2392–2404.
Giuffrè M, Colecchia A, Crocè LS. Elastography: where are we now? Minerva Gastroenterol. 2021. https://doi.org/10.23736/S2724-5985.20.02773-7
Franchis de R, Bosch J, Garcia-Tsao G. Baveno VII—renewing consensus in portal hypertension. J Hepatol. 2022;76:959–974.
Rodrigues SG, Mendoza YP, Bosch J. Beta-blockers in cirrhosis: evidence-based indications and limitations. JHEP Rep. 2020;2:100063.
Choi S-Y , Jeong WK, Kim Y. Shear-wave elastography: a noninvasive tool for monitoring changing hepatic venous pressure gradients in patients with cirrhosis. Radiology. 2014;273:917–926.
Binzberger A, Hänle M, Pfahler M. Spleen and liver stiffness evaluation by ARFI imaging: a reliable tool for a short-term monitoring of portal hypertension? Int J Hepatol. 2022;2022:1–14.
Marasco G, Dajti E, Ravaioli F. Spleen stiffness measurement for assessing the response to β-blockers therapy for high-risk esophageal varices patients. Hepatol Int. 2020;14:850–857.
Kim HY, So YH , Kim W. Non-invasive response prediction in prophylactic carvedilol therapy for cirrhotic patients with esophageal varices. J Hepatol. 2019;70:412–422.
Aubé C, Bazeries P, Lebigot J. Liver fibrosis, cirrhosis, and cirrhosis-related nodules: Imaging diagnosis and surveillance. Diagn Interv Imaging. 2017;98:455–468.
Ferraioli G, Roccarina D. Update on the role of elastography in liver disease. Therap Adv Gastroenterol. 2022;15:175628482211406.
Pugh RNH , Murray-Lyon IM, Dawson JL. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 2005;60:646–649.
Kamath PS, Kim WR. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797–805.
De Franchis R, Abraldes JG, Bajaj J, et al. Expanding consensus in portal hypertension Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;743–752.
Abby C, Philips Sahney A. Oesophageal and gastric varices: historical aspects, classification and grading: everything in one place. Gastroenterol Rep (Oxf). 2016;4:186–195.
Giuffrè M, Macor D, Masutti F. Evaluation of spleen stiffness in healthy volunteers using point shear wave elastography. Ann Hepatol. 2019 ;18:736–741.
Giuffrè M, Macor D, Masutti F. Spleen Stiffness Probability Index (SSPI): a simple and accurate method to detect esophageal varices in patients with compensated liver cirrhosis. Ann Hepatol. 2020;19:53–61.
Giuffrè M, Fouraki S, Comar M. The Importance of transaminases flare in liver elastography: characterization of the probability of liver fibrosis overestimation by hepatitis C virus-induced cytolysis. Microorganisms. 2020;8:348.
Giuffrè M, Giuricin M, Bonazza D. Optimization of point-shear wave elastography by skin-to-liver distance to assess liver fibrosis in patients undergoing bariatric surgery. Diagnostics. 2020;10:795.
Giuffrè M, Bedogni G, Abazia C. Spleen stiffness can be employed to assess the efficacy of spontaneous portosystemic shunts in relieving portal hypertension. Ann Hepatol. 2020;19:691–693.
Giuffrè M, Fouraki S, Campigotto M. Alanine aminotransferase and spleno-portal dynamics affect spleen stiffness measured by point shear-wave elastography in patients with chronic hepatitis C in the absence of significant liver fibrosis. J Ultrasound. 2021;24:67–73.
Barr RG , Wilson SR, Rubens D. Update to the Society of radiologists in ultrasound liver elastography consensus statement. Radiology. 2020;296:263–274.
Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika. 1965;52:591–611.
Najmi A, Sadasivam B, Ray A. How to choose and interpret a statistical test? An update for budding researchers. J Family Med Prim Care. 2021;10:2763.
Tibshirani R. Regression shrinkage and selection via the lasso. J Roy Stat Soc: Ser B (Methodol). 1996;58:267–288.
Reiberger T, Ferlitsch A, Payer BA. Non-selective β-blockers improve the correlation of liver stiffness and portal pressure in advanced cirrhosis. J Gastroenterol. 2012;47:561–568.
Zacharias AP, Jeyaraj R, Hobolth L. Carvedilol versus traditional, non-selective beta-blockers for adults with cirrhosis and gastroesophageal varices. Cochr Database Syst Rev. 2018. https://doi.org/10.1002/14651858.CD011510.pub2.
Acknowledgements
NSBB-Elasto-Response-Prediction Group: Mauro Giuffrè, Johannes Dupont, Alessia Visintin, Flora Masutti, Cristiana Abazia, Clara Faini, Michele Campigotto, Francesca Dottor, Marco Gulotta, Irma Valeria Albergati, Fabio Monica, Kisung You, Dennis L. Shung, Lory Saveria Crocè
Funding
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Mauro Giuffrè, Johannes Dupont, Alessia Visintin, Flora Masutti, Fabio Monica, Kisung You, Dennis L. Shung, Lory Saveria Crocè have no conflict of interest to declare.
Ethical approval
The study was conducted in accordance with the ethical principles for medical research involving human subjects as indicated by the Declaration of Helsinki. The study was carried out following the guidelines of the local Ethics Committee for conducting research involving humans (Protocol Number: 2783).
Informed consent
Informed consent was obtained for each patient participating in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The collaborators of “The NSBB-Elasto-Response-Prediction Group” are listed in the acknowledgements.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giuffrè, M., Dupont, J., Visintin, A. et al. Predicting response to non-selective beta-blockers with liver–spleen stiffness and heart rate in patients with liver cirrhosis and high-risk varices. Hepatol Int (2024). https://doi.org/10.1007/s12072-024-10649-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12072-024-10649-7