Abstract
Introduction
Current guidelines discourage the use of direct-acting antiviral (DAA) containing protease-inhibitor (PI) in advanced HCV cirrhosis. We aimed to compare the real-world tolerability of PI vs. non-PI DAA regimens in this population.
Methods
We identified advanced cirrhosis patients treated with DAA from the REAL-C registry. The primary outcome was significant worsening or improvement in CPT or MELD scores following DAA treatment.
Results
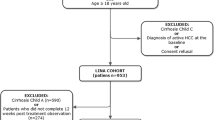
From the REAL-C registry of 15,837 patients, we included 1077 advanced HCV cirrhosis patients from 27 sites. 42% received PI-based DAA. Compared to non-PI group, the PI group was older, had higher MELD and higher percentage with kidney disease. Inverse probability of treatment weighting (IPTW; matching on age, sex, history of clinical decompensation, MELD, platelet, albumin, Asia site, Asian ethnicity, hypertension, hemoglobin, genotype, liver cancer, ribavirin) was used to balance the two groups. In the IPTW-matched cohorts, the PI and non-PI groups had similar SVR12 (92.9% vs. 90.7%, p = 0.30), similar percentages of significant worsening in CTP or MELD scores at posttreatment week 12 and 24 (23.9% vs. 13.1%, p = 0.07 and 16.5% vs. 14.6%, p = 0.77), and similar frequency of new HCC, decompensating event, and death by posttreatment week 24. In multivariable analysis, PI-based DAA was not associated with significant worsening (adjusted odds ratio = 0.82, 95% CI 0.38–1.77).
Conclusion
Tolerability and treatment outcomes were not significantly different in advanced HCV cirrhosis treated with PI-based (vs. non-PI) DAA up to CTP-B or MELD score of 15. Safety of PI-based DAA in those with CTP-C or MELD beyond 15 awaits further data.



Similar content being viewed by others
Data availability
Due to privacy policy, data are not publicly available.
Abbreviations
- DAA:
-
Direct-acting antivirals
- HCV:
-
Hepatitis C virus
- SVR:
-
Sustained virologic response
- PI:
-
Protease inhibitors
- MELD:
-
Model for end-stage-liver disease
- CTP:
-
Child–Turcotte–Pugh
- IPTW:
-
Inverse probability of treatment weighting
- REAL-C:
-
Real-World Evidence from the Asia Pacific Rim Liver Consortium for HCV
- USA:
-
United States of America
- HE:
-
Hepatic encephalopathy
- HCC:
-
Hepatocellular carcinoma
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- SD:
-
Standard deviation
References
Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol 2022;7(5):396–415
Kiser JJ. Safety of hepatitis C viral protease inhibitors in compensated cirrhotic: lingering concerns put to rest? Clin Infect Dis 2019;69(10):1665–1666
https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-occurrence-serious-liver-injury-use-hepatitis-c-medicines-mavyret-zepatier-and Assessed on 1 Mar 2023
AASLD-IDSA HCV Guidance Panel. Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis 2018;67(10):1477–1492
EASL Governing Board representative. Panel members: EASL recommendations on treatment of hepatitis C: final update of the series☆. J Hepatol 2020;73(5):1170–1218
Kosloski MP, Wang H, Pugatch D, et al. Pharmacokinetics and safety of glecaprevir and pibrentasvir in HCV-negative subjects with hepatic impairment. Eur J Clin Pharmacol 2019;75(2):217–226. https://doi.org/10.1007/s00228-018-2576-4
Banerjee D, Reddy KR. Review article: safety and tolerability of direct-acting anti-viral agents in the new era of hepatitis C therapy. Aliment Pharmacol Ther 2016;43:674–696
Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis C virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology 2017;152(1372–82): e2
Forns X, Lee SS, Valdes J, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infections in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis 2017;17:1062–1068
Gane E, Poordad F, Zadeikis N, et al. Safety and pharmacokinetics of glecaprevir/pibrentasvir in adults with chronic genotype 1–6 hepatitis C virus infections and compensated liver disease. Clin Infect Dis 2019;69(10):1657–1664
Maan R, van Tilborg M, Deterding K, et al. Safety and effectiveness of direct-acting antiviral agents for treatment of patients with chronic hepatitis C Virus infection and cirrhosis. Clin Gastroenterol Hepatol 2016;14(12):1821-1830.e6
Torgersen J, Newcomb CW, Carbonari DM, et al. Protease inhibitor-based direct-acting antivirals are associated with increased risk of aminotransferase elevations but not hepatic dysfunction or decompensation. J Hepatol 2021;75(6):1312–1322. https://doi.org/10.1016/j.jhep.2021.07.021
Berkan-Kawińska A, Piekarska A, Janczewska E, et al. Real-world effectiveness and safety of direct-acting antivirals in patients with cirrhosis and history of hepatic decompensation: Epi-Ter2 Study. Liver Int 2021;41(8):1789–1801. https://doi.org/10.1111/liv.14858
Wong YJ, Nguyen MH. Is it safe to treat chronic hepatitis C patients with decompensated cirrhosis with protease inhibitor-based DAA? J Hepatol 2022;S0168–8278(22):00008–00013. https://doi.org/10.1016/j.jhep.2021.12.037
Wong YJ, Nguyen MH. Is it safe to treat chronic hepatitis C patients with decompensated cirrhosis with protease inhibitor-based DAA? Liver Int 2022. https://doi.org/10.1111/liv.15214.10.1111/liv.15214
Tanaka Y, Ogawa E, Huang CF, et al. HCC risk post-SVR with DAAs in East Asians: findings from the REAL-C cohort. Hepatol Int 2020;14(6):1023–1033. https://doi.org/10.1007/s12072-020-10105-2
Verna EC, Morelli G, Terrault NA, et al. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: Real-world experience from HCV-TARGET cohort. J Hepatol 2020;73(3):540–548. https://doi.org/10.1016/j.jhep.2020.03.031
Chesnaye NC, Stel VS, Tripepi G, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J 2021;15(1):14–20. https://doi.org/10.1093/ckj/sfab158
Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-ft. tests for the logistic regression model. Stat Med 1997;16:965–980
D’Amico G, Morabito A, D’Amico M, Pasta L, Malizia G, Rebora P, et al. Clinical states of cirrhosis and competing risks. J Hepatol 2018;68(3):563–576
Curry MP, Oleary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med 2015;373(27):2618–2628
Welzel TM, Petersen J, Herzer K, Ferenci P, Gschwantler M, Wedemeyer H, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut 2016;65(11):1861–1870
Acknowledgements
We would like to acknowledge Kaohsiung Medical University Research Center Grant: Center for Liquid Biopsy and Cohort Research KMHK-DK(C)111006 and KMHK-DK(C)111004 for supporting Dr. Ming-Lung Yu.
Funding
The study is supported in part by an investigator-initiated research grant from Gilead Sciences to Stanford University. The funding body has no role in study design, data collection, data interpretation, manuscript writing and the decision to publish.
Author information
Authors and Affiliations
Contributions
Guarantor of the article: MHN. Specific author contributions: study design: YJW, ST, and MHN. Data collection: all the authors. Data analysis: ST, YJW and MHN. Data interpretation: all the authors. Drafting of the article: YJW, ST and MHN. Study concept and study supervision: MHN.
Corresponding author
Ethics declarations
Conflict of interest
WYJ: Speaker: Gilead and AbbVie; MHN: research support: Pfizer, Enanta, Gilead, Exact Sciences, Vir Biotech, Helio Health, National Cancer Institute, Glycotest, B.K. Kee Foundation; Consulting and/or Advisory Board: Intercept, Exact Science, Gilead, GSK, Eli Lilly, Laboratory of Advanced Medicine, Janssen; AN: Speaker fee and research grant: Gilead and AbbVie; LSG: Advisory Board: Gilead Sciences, Roche, GSK, Janssen, Grifols, Assembly, Arbutus, Abbott, Sysmex; Speakers Bureau: Gilead Sciences, Abbott, Janssen; Educational/research funding: Abbott, Merck Sharpe and Dohme, Gilead Sciences, Sysmex GW: Speakers’ fees: Abbott, Abbvie, Ascletis, Bristol-Myers Squibb, Echosens, Gilead Sciences, Janssen and Roche; Research grant: Gilead Sciences; MA: Speakers’ fees: AbbVie, Gilead; Research grant: AbbVie; CHT: Speaker: Abbvie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and Bayer; HT: Abbie, Astellas Pharma and Sysmex Corporation; KT: Speaker: Abbie; EO: Speakers’ fees: Abbvie, Gilead Sciences; Research grant: Gilead Sciences; WLC: Speaker: Gilead, AbbVie, BMS, PharmaEssentia; Member of Advisory Board: Gilead, AbbVie, BMS, Pharma Essentia; HT: AbbVie, Gileads, Bayer, Eisai, MSD, Janssen; MFY: Advisory board member/consultant for and/or received research funding: AbbVie, Aligos Therarpeutics, AiCuris, Antios Therapeutics, Arrowhead Pharmaceuticals, Arbutus Biopharma, Assembly Biosciences, Bristol Myer Squibb, Bluejay Therapeutics, Clear B Therapeutics, Dicerna Pharmaceuticals, Finch Therapeutics, Fujirebio Incorporation, GlaxoSmithKline, Gilead Sciences, Immunocore, Janssen, Merck Sharp and Dohme, and Hoffmann-La Roche, Vir Biotechnology; YML: Consultant: Abbvie, Abbott, BMS, Gilead, Merck and Roche diagnostics; Speaker: Abbvie, Abbott, BMS, Gilead, IPSEN, Merck and Roche; YU: Speakers’ fees: Abbvie Inc, Research grant: Gilead Sciences, Abbvie; ME: Speakers’ fee: AbbVie; FJ: Speaker fees: Gilead Sciences, MSD and Ascletis, Consulting or advisory board: Gilead Sciences and MSD; YT: Speakers’ fee: Gilead Sciences, Fujirebio Inc, AbbVie, Research grants: Janssen, Gilead, Board of Trustees of the Leland Stanford Junior University, AbbVie.
Ethical approval
All the procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted and ethical approval was obtained from Stanford University, Stanford, California, USA and each participating site.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wong, Y.J., Tran, S., Huang, CF. et al. Real-world treatment outcome with protease inhibitor direct-acting antiviral in advanced hepatitis C cirrhosis: a REAL-C study. Hepatol Int 17, 1150–1161 (2023). https://doi.org/10.1007/s12072-023-10547-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10547-4