Abstract
Background and aims
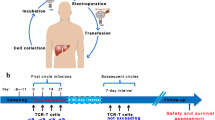
Liver transplantation (LT) is the primary curative option for cirrhotic patients with early-stage hepatocellular carcinoma (HCC). However, tumor recurrence occurs in 15–20% of cases with unfavorable prognosis. We have developed a library of T cell receptors (TCRs) specific for different hepatitis B virus (HBV) antigens, restricted by different molecules of human leucocyte antigen (HLA)-class I, to redirect T cells against HBV antigens (Banu in Sci Rep 4:4166, 2014). We further demonstrated that these transiently functional T cells specific for HBV obtained through messenger RNA (mRNA) electroporation can eliminate HCC cells expressing HBV antigens in vitro and in vivo (Kah in J Clin Invest 127:3177–3188, 2017). A phase I clinical trial for patients with HCC recurrence post-liver transplant was conducted to assess the safety, tolerability, and anti-tumor efficacy of transiently functional HBV-TCR T cells. Here, we report the clinical findings with regard to the safety and anti-tumor efficacy of mRNA electroporated HBV-specific TCR-T cells. (ClinicalTrials.gov identifier: NCT02719782).
Patients and methods
A total of six patients with HBV-positive recurrent HCC post-liver transplant and HLA-matched to TCR targeting hepatitis B surface antigen (HBsAg) or hepatitis B core antigen (HBcAg) (HLA-A*02:01/HBsAg, HLA-A*11:01/HBcAg, HLA-B*58:01/HBsAg or HLA-C*08:01/HBsAg) were enrolled in this study. The primary objective was to assess the safety of short-lived mRNA electroporated HBV-TCR T cells based on the incidence and severity of the adverse event (AE) graded per National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), Version 4.0. The secondary objective was to determine the effectiveness of HBV-TCR T cells as per RECIST 1.1 criteria. Patients were followed up for survival for 2 years post-end of treatment.
Results
The median age of the six patients was 35.5 years (range: 28–47). The median number of HBV-TCR T cell infusions administered was 6.5 (range: 4–12). The treatment-related AE included grade 1 pyrexia. This study reported no cytokine release syndrome nor neurotoxicity. One patient remained alive and five were deceased at the time of the data cutoff (30 April 2020).
Conclusion
This study has demonstrated that multiple infusions of mRNA electroporated HBV-specific TCR T cells were well-tolerated in patients with HBV-positive recurrent HCC post-liver transplant.



Similar content being viewed by others
Data availability
Supporting data for this study are available from the corresponding authors and the first authors upon reasonable request.
Abbreviations
- AE:
-
Adverse event
- ACT:
-
Adoptive cell transfer
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- BW:
-
Body weight
- BCLC:
-
Barcelona clinic liver cancer
- CAR-T:
-
Chimeric antigen receptor-T
- CIK:
-
Cytokine-induced killer
- CnB:
-
Calcineurin B
- CTCAE:
-
Common terminology criteria for adverse events
- ECOG:
-
European cooperative oncology group
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HLA:
-
Human leukocyte antigen
- HBcAg:
-
Hepatitis B core antigen
- HBsAg:
-
Hepatitis B surface antigen
- HBeAg:
-
Hepatitis B e antigen
- IMPDH:
-
Inosine- 5′- monophosphate dehydrogenase
- LT:
-
Liver transplantation
- mRNA:
-
Messenger RNA
- MMF:
-
Mycophenolate mofetil
- MedDRA:
-
Medical dictionary for regulatory activities terminology
- NCI:
-
National cancer institute
- OS:
-
Overall survival
- ORR:
-
Objective response rate
- PD:
-
Progressive disease
- PBMC:
-
Peripheral blood mononuclear cell
- SD:
-
Stable disease
- TAC:
-
Tacrolimus
- TCR:
-
T cell receptor
- TNM:
-
Tumor node metastasis
- TTP:
-
Time to progression
References
Banu N, et al. Building and optimizing a virus-specific T cell receptor library for targeted immunotherapy in viral infections. Sci Rep. 2014;4:4166
Kah J, et al. Lymphocytes transiently expressing virus-specific T cell receptors reduce hepatitis B virus infection. J Clin Invest. 2017;127(8):3177–3188
Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64(1 Suppl):S84-s101
Colquhoun SD. Hepatocellular carcinoma clinical update: Current standards and therapeutic strategies. Liver Research. 2020;4(4):180–190
Ashtari S, et al. Hepatocellular carcinoma in Asia: prevention strategy and planning. World J Hepatol. 2015;7(12):1708–1717
Lombardi A, et al. Hepatocarcinoma: genetic and epigenetic features. Minerva Gastroenterol Dietol. 2018;64(1):14–27
Orcutt ST, Anaya DA. Liver resection and surgical strategies for management of primary liver cancer. Cancer Control. 2018;25(1):1073274817744621
Silva MF, et al. Liver resection and transplantation offer similar 5-year survival for child-pugh-turcotte a HCC-patients with a single nodule up to 5 cm: a multicenter, exploratory analysis. Eur J Surg Oncol. 2013;39(4):386–395
Wong RJ, et al. Primary surgical resection versus liver transplantation for transplant-eligible hepatocellular carcinoma patients. Dig Dis Sci. 2014;59(1):183–191
Lee JH, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148(7):1383–91.e6
Jia CC, et al. Efficacy of cytokine-induced killer cell-based immunotherapy for hepatocellular carcinoma. Am J Cancer Res. 2019;9(6):1254–1265
Ma Y, et al. Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Exp Hematol Oncol. 2012;1(1):11
Watanabe K, Nishikawa H. Engineering strategies for broad application of TCR-T- and CAR-T-cell therapies. Int Immunol. 2021;33(11):551–562
Shi D, et al. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clin Cancer Res. 2020;26(15):3979–3989
Tan AT, et al. Immunological alterations after immunotherapy with short lived HBV-TCR T cells associates with long-term treatment response in HBV-HCC. Hepatol Commun. 2022;6(4):841–854
Roddy H, Meyer T, Roddie C. Novel cellular therapies for hepatocellular carcinoma. Cancers (Basel). 2022;14(3):504
Manfredi F, et al. TCR redirected T cells for cancer treatment: achievements, hurdles, and goals. Front Immunol. 2020;11:1689
Zhao Q, et al. Engineered TCR-T cell immunotherapy in anticancer precision medicine: pros and cons. Front Immunol. 2021;12: 658753
Tan AT, et al. Use of expression profiles of HBV-DNA integrated into genomes of hepatocellular carcinoma cells to select T cells for immunotherapy. Gastroenterology. 2019;156(6):1862-1876.e9
Hafezi M, Bertoletti A, Tan A. Personalized T-cell therapy in liver transplanted patients with hepatitis B virus related hepatocellular carcinoma. Hepatoma Research. 2020;6:23
Tambur AR. Human leukocyte antigen matching in organ transplantation: what we know and how can we make it better (Revisiting the past, improving the future). Curr Opin Organ Transplant. 2018;23(4):470–476
Neumann UP, et al. Impact of human leukocyte antigen matching in liver transplantation. Transplantation. 2003;75(1):132–137
Wohlleber D, Knolle PA. The liver as an immune-privileged site. In Stein-Streilein J, editor., Infection, immune homeostasis and immune privilege. Basel: Springer Basel; 2012. 93–106
Patel YA, et al. The impact of human leukocyte antigen donor and recipient serotyping and matching on liver transplant graft failure in primary sclerosing cholangitis, autoimmune hepatitis, and primary biliary cholangitis. Clin Transplant. 2018;32(10):e13388–e13388
Mahawar KK, Bal AM. Role of HLA matching in liver transplant. Transplantation. 2004;78(2):643
Muro M, et al. Effect of HLA matching on liver graft survival. Transpl Proc. 1999;31(6):2477–2479
Hafezi M, et al. Immunosuppressive drug-resistant armored T-cell receptor T cells for immune therapy of HCC in liver transplant patients. Hepatology. 2021;74(1):200–213
Zhang D, et al. Preoperative serum hepatitis B virus DNA was a risk factor for hepatocellular carcinoma recurrence after liver transplantation. Ann Med. 2022;54(1):2213–2221
Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16(9):566–581
Koh S, et al. A practical approach to immunotherapy of hepatocellular carcinoma using T cells redirected against hepatitis B virus. Mol Ther Nucleic Acids. 2013;2: e114
Yoon JS, et al. Adjuvant cytokine-induced killer cell immunotherapy for hepatocellular carcinoma: a propensity score-matched analysis of real-world data. BMC Cancer. 2019;19(1):523
Acknowledgements
The authors would like to thank Antonio Bertoletti and Anthony T. Tan for their contribution to the development of the investigational HBV-TCR T cell product and clinical trial design. The authors would also like to thank the participants who contributed to this study.
Funding
This work was supported by a project funded by the National Natural Science Foundation of China (82260110, 81870449, 82170674, 51933011), China Postdoctoral Science Foundation (2019M653904XB), Natural Science Foundation of Xinjiang Uyghur Autonomous Region (2020D01C006). Science and Technology Projects in Guangzhou (202102010310).
Author information
Authors and Affiliations
Contributions
LW and SK developed the investigational HBV-TCR T cell product. TW, QZ and WC designed and supervised the clinical trial. JL, XC, LY and WC contributed to the manufacturing of HBV-TCR T cells. FY, WC, JC, PL, CD and YC managed the patients. XZ, TW and RWW performed data analysis. RWW and FY wrote the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Regina Wanju Wong, Lu-En Wai, Sarene Koh, and Tingting Wang are employees of Lion TCR Pte Ltd. Fan Yang, Xiaofang Zheng, Jianxi Lu, Jintao Cheng, Panlong Li, Cong Du, Yunhao Chen, Xiaoyan Chen, Li Yang, Wanxin Chen, Qi Zhang, and Wenjie Chen have no conflicts of interest to disclose.
Ethics approval and consent to participate
All participants provided informed consent, and the study was approved by the ethics committee of the third affiliated hospital of Sun Yat-sen University.
Consent for publication
Each author has given his/her consent to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, F., Zheng, X., Koh, S. et al. Messenger RNA electroporated hepatitis B virus (HBV) antigen-specific T cell receptor (TCR) redirected T cell therapy is well-tolerated in patients with recurrent HBV-related hepatocellular carcinoma post-liver transplantation: results from a phase I trial. Hepatol Int 17, 850–859 (2023). https://doi.org/10.1007/s12072-023-10524-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10524-x