Abstract
Background
Although the elderly constitute more than a third of hepatocellular carcinoma (HCC) patients, they have not been adequately represented in treatment and prognosis studies. Thus, there is not enough evidence to guide the treatment of such patients. The objective of this study is to identify the prognostic factors of older patients with HCC and to construct a new prognostic model for predicting their overall survival (OS).
Methods
2,721 HCC patients aged ≥ 65 were extracted from the public database-Surveillance, Epidemiology, and End Results (SEER) and randomly divided into a training set and an internal validation set with a ratio of 7:3. 101 patients diagnosed from 2008 to 2017 in the First Affiliated Hospital of Zhejiang University School of Medicine were identified as the external validation set. Univariate cox regression analyses and multivariate cox regression analyses were adopted to identify these independent prognostic factors. A predictive nomogram-based risk stratification model was proposed and evaluated using area under the receiver operating characteristic curve (AUC), calibration curves, and a decision curve analysis (DCA).
Results
These attributes including age, sex, marital status, T stage, N stage, surgery, chemotherapy, tumor size, alpha-fetoprotein level, fibrosis score, bone metastasis, lung metastasis, and grade were the independent prognostic factors for older patients with HCC while predicting survival duration. We found that the nomogram provided a good assessment of OS at 1, 3, and 5 years in older patients with HCC (1-year OS: (training set: AUC = 0.823 (95%CI 0.803–0.845); internal validation set: AUC = 0.847 (95%CI 0.818–0.876); external validation set: AUC = 0.732 (95%CI 0.521–0.943)); 3-year OS: (training set: AUC = 0.813 (95%CI 0.790–0.837); internal validation set: AUC = 0.844 (95%CI 0.812–0.876); external validation set: AUC = 0.780 (95%CI 0.674–0.887)); 5-year OS: (training set: AUC = 0.839 (95%CI 0.806–0.872); internal validation set: AUC = 0.800 (95%CI 0.751–0.849); external validation set: AUC = 0.821 (95%CI 0.727–0.914)). The calibration curves showed that the nomogram was with strong calibration. The DCA indicated that the nomogram can be used as an effective tool in clinical practice. The risk stratification of all subgroups was statistically significant (p < 0.05). In the stratification analysis of surgery, larger resection (LR) achieved a better survival curve than local destruction (LD), but a worse one than segmental resection (SR) and liver transplantation (LT) (p < 0.0001). With the consideration of the friendship to clinicians, we further developed an online interface (OHCCPredictor) for such a predictive function (https://juntaotan.shinyapps.io/dynnomapp_hcc/). With such an easily obtained online tool, clinicians will be provided helpful assistance in formulating personalized therapy to assess the prognosis of older patients with HCC.
Conclusions
Age, sex, marital status, T stage, N stage, surgery, chemotherapy, tumor size, AFP level, fibrosis score, bone metastasis, lung metastasis, and grade were independent prognostic factors for elderly patients with HCC. The constructed nomogram model based on the above factors could accurately predict the prognosis of such patients. Besides, the developed online web interface of the predictive model provide easily obtained access for clinicians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary liver cancer is the sixth most common malignant tumor and the fourth leading cause of cancer-related deaths worldwide [1, 2]. In 2020, 907,100 new cases of primary liver cancer were reported, and 8.3% of patients died of it [3]. Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer [4]. It usually occurs in those patients who suffer from chronic inflammation and fibrosis caused by viral hepatitis, alcohol, and metabolic-related fatty liver disease [5]. HCC’s 5-year overall survival (OS) rate is often less than 20% [6]. Tumor resection is the most effective treatment for early stage HCC, but the recurrence and metastasis rates are high and the prognosis of patients with HCC is usually poor [7, 8].
Improvements in the treatment of chronic liver diseases and the extension of life expectancy have resulted in an increase in the number of elderly patients with HCC. Although the elderly constitute more than a third of HCC patients, they have not been adequately represented in treatment and prognosis studies. Thus, there is not enough evidence to guide the treatment of such patients [9, 10]. The incidence rate of HCC is estimated to increase by approximately 59% by 2030, whereas individuals aged 65 or above are expected to constitute over 50% HCC patient by then [11].
Nomogram is considered a widely used predictive model for evaluating the prognosis of cancer patients [12,13,14]. In this study, we aim to identify the prognostic factors of elderly patients with HCC and construct a new prognostic model for predicting their OS, which would facilitate the provision of therapy suggestions and assist clinical decision-making.
Materials and methods
Subject selection
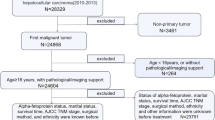
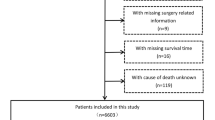
The Surveillance, Epidemiology, and End Results (SEER) is a dominant cancer statistics database in the United States (US) [15]. The database contains the diagnosis, treatment, and survival data of millions of cancer patients in US and other countries. The data regarding HCC patients during 2010–2015 were selected and handled by SEER*Stat 8.3.9 (https://seer.cancer.gov/) in this study. The inclusion and exclusion criteria are outlined in Supplementary Fig. 1. To be included, the patients must match the criteria: (1) diagnosed with HCC between 2010 and 2015 and (2) 65 years or older. Contrarily, we excluded: (1) patients for whom liver cancer was not their first primary tumor, (2) those with unknown alpha-fetoprotein (AFP) level, (3) those without follow-up time and (4) those with missing survival data. Moreover, we retrospectively enrolled elderly patients diagnosed with HCC to construct the external validation set between 2008 and 2017 from the First Affiliated Hospital of Zhejiang University School of Medicine in light of the selection criteria. The protocol of this research was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (Ethical approval No. IIT20230048B).
Variables selection
Fourteen variables were selected in this study: age at diagnosis, race, sex, marital status, T stage, N stage, surgery, radiotherapy, chemotherapy, tumor size, AFP level, bone metastasis, lung metastasis, and grade. Fibrosis score (FS) was also adopted in this study. Surgery was divided into five categories: no surgery, local destruction (LD), segmental resection (SR), larger resection (LR), and liver transplantation (LT). For LD patients, there exists a large range of therapeutic options including photodynamic therapy (PDT), electrocautery / fulguration (includes the use of hot forceps for tumor destruction), cryosurgery, laser, alcohol (percutaneous ethanol injection [PEI]), heat-radio-frequency ablation (RFA) and other methods (e.g., ultrasound, acetic acid). The above therapeutic options have been defined in SEER database. Considering that age and tumor size were continuous variables, X-tile was used to determine the optimal cutoff values for them [16]. The results showed that the best cutoff values for age were 74 and 80, whereas the best cutoff values for tumor size were 5.6 and 8.5 cm. OS was the outcome of the model proposed in this study, which was defined as the time from randomization until death from any cause or the date of the last follow-up.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 and R (version 4.0.2, Vienna, Austria). Values were considered statistically significant at p < 0.05. Univariate cox regression analysis and multivariate cox regression analysis were adopted to identify independent prognostic factors. The risk ratio (HR) and 95% confidence interval (CI) were used to show the impact of the variables on the patients’ survival. A nomogram was constructed based on these independent prognostic factors. The discriminatory values of the models were evaluated based on the concordance index (C-index). The area under the curve (AUC) of the receiver operating characteristic (ROC) curve was used to evaluate the prognostic accuracy of the nomogram. Calibration curve was generated to evaluate the calibration of the nomogram. In addition, a decision curve analysis (DCA) was performed to demonstrate the clinical benefit of the nomogram [17]. To streamline the power calculation estimation, we produced PowerTools—an interactive open-source web application that was written in R using the Shiny framework (http://www.shinyapps.io/).
In addition, we developed a risk stratification model based on the total score of each patient, as calculated by the nomogram. Then, X-Tile was used to determine the best cutoff value to divide patients into low-risk, intermediate-risk, and high-risk groups. Kaplan–Meier curves and log-rank tests were used to analyze and compare the OS of patients in different subgroups.
Results
Patient characteristics
A total of 2,721 patients extracted from the SEER database were divided into a training set (N = 1904) and an internal validation set (N = 817). The chi-square test showed that there was no significant difference between the two sets (Table 1). In the training set, 58.4% (1112/1904), 27.2% (518/1,904), and 14.4% (274/1904) of the patients were aged < 74, 74–80, and > 80, respectively. In addition, 52.0% (990/1904), 21.8% (415/1904), and 26.2% (499/1904) of the patients’ tumor sizes were < 5.6 cm, 5.6–8.5 cm, and > 8.5 cm, respectively. Furthermore, 62.9% (1197/1904) of the patients had elevated AFP levels, whereas 37.1% (707/1,904) of them had normal AFP levels. Patient characteristics of the external validation set (N = 101) was listed in Supplementary Table 1.
Univariate and multivariate analysis for prognosis
To identify OS-related variables, 15 variables were used in the univariate cox analysis. The univariate cox regression analysis showed that all variables were statistically significant (p < 0.05). The multivariate cox analysis indicated that higher age, sex (male), being unmarried, higher T stage, higher N stage, no surgery, no chemotherapy, larger tumor size, elevated AFP, higher fibrosis score, bone metastasis, lung metastasis, and higher grade were all independently associated with poor OS of HCC patients, as shown in Table 2 and Fig. 1.
Prognostic nomogram model
A nomogram was constructed based on all independent OS-related factors from the training set (as shown in Fig. 2), and then scores were assigned to the clinical variables in each subgroup (as shown in Table 3). Meanwhile, the time-dependent ROC curves showed that the AUC values at 1, 3, and 5 years were 0.823(95%CI 0.803–0.845), 0.813 (95%CI 0.790–0.837), and 0.839 (95%CI 0.806–0.872), respectively. This suggested favorable discrimination of the nomogram (as shown in Fig. 3). In the internal validation set, the AUC values at 1, 3, and 5 years were 0.847 (95%CI 0.818–0.876), 0.844 (95%CI 0.812–0.876), and 0.800 (95%CI 0.751–0.849), respectively. In the external validation set, the AUC values at 1, 3, and 5 years were 0.732 (95%CI 0.521–0.943), 0.780 (95%CI 0.674–0.887), and 0.821 (95%CI 0.727–0.914), respectively (as shown in Supplementary Fig. 2).
Moreover, the calibration curves (bootstraps = 1000) of the training set and internal validation set indicated that the nomogram had a strong calibration (as shown in Fig. 4). In addition, we compared the clinical practicability of the nomogram and the TNM staging system using DCA. The results indicated that the nomogram had a better clinical benefit and a larger threshold probability range, which confirmed that the nomogram can be used as an effective tool in clinical practice (as shown in Fig. 5).
Risk stratification model
Based on the constructed prognostic nomogram model, the patients were divided into the low-risk group (1,553/2,721, 57.1%; total score < 83), intermediate-risk group (846/2,721, 31.1%; total score 83–113), and high-risk group (322/2,721, 11.8%; total score > 113). The results of the Kaplan–Meier survival analysis with log-rank tests showed that there were different survival patterns among patients in the three groups. The prognosis of patients in the low-risk group was significantly better than that of those in the high-risk group (p < 0.0001) (as shown in Fig. 6).
Risk stratification for subgroup analysis
Although the constructed nomogram model worked well in the training and internal validations, its effectiveness in the subgroups was unclear. Therefore, we divided these patients into different subgroups according to the age, tumor size, T stage, grade, AFP level and surgery, to further confirm the effectiveness of the nomogram. The results indicated that in both the training and internal validations, the risk stratification of all subgroups was statistically significant (p < 0.05). This implied that the nomogram was effective for the distinction of the prognosis of different subgroups of HCC patients (as shown in Figs. 7, 8, and 9).
Stratification analysis of surgery
To further determine whether more radical surgeries should be taken for the older patients, we also performed surgical stratification analysis based on the univariate OS Cox model (age, T stage, tumor size, fibrosis score and grade). The results showed that LT had the best survival duration in the < 74 age band: HR 0.165 (95%CI 0.116–0.236), and unexpectedly, LR did not show better survival duration than SR in all ages ranges [< 74: HR 0.257 (95%CI 0.202–0.328) vs. HR 0.191 (95%CI 0.151–0.241); 74–80: HR 0.316 (95%CI 0.237–0.423) vs. HR 0.244 (95%CI 0.178–0.335); > 80: HR 0.322 (95%CI 0.205–0.507) vs. HR 0.169 (95%CI 0.092–0.312)]. Meanwhile, SR had the best survival duration in the 0–4 FS band: HR 0.215 (95%CI 0.132–0.351), LT had the best survival duration in the 5–6 FS band: HR 0.126 (95%CI 0.073–0.218) (as shown in Table 4).
Moreover, a more detailed plot was conducted on OS. It was shown that both in training set and internal validation, the surgery group had a higher survival curve than the non-surgery group did, and LT had the highest survival curve (p < 0.0001) (as shown in Supplementary Fig. 3). LR had a better survival curve than LD but a worse one than SR and LT (p < 0.0001).
Online service of the constructed nomogram model
To provide easily obtained access of the proposed model, we developed an online website (https://juntaotan.shinyapps.io/dynnomapp_hcc/) to provide the service of nomogram model for medical staff. The provided function could automatically receive and calculate a patient’s survival probability. The scoring module enables the early identification of high-risk patients, which could further facilitate the appropriate treatment to prolong the survival time.
Discussion
A total of 2,822 older patients with HCC were selected in this study. The univariate and multivariate cox regression analysis successfully identified 13 independent prognostic factors including age, sex, marital status, T stage, N stage, surgery, chemotherapy, tumor size, AFP level, fibrosis score, bone metastasis, lung metastasis, and grade. The prognostic nomogram model was constructed based on these factors. The C-index, calibration curve, and DCA evaluated the model based on discrimination, calibration, and clinical usefulness, respectively. In addition, we constructed a risk stratification model based on the total score of each patient provided by the nomogram.
In 2020, through univariate and multivariate Cox analyses, Liu and colleagues developed a full age spectrum prognosis model that included 6 predictors to evaluate the prognosis of HCC patients [18]. However, we considered that it is impossible to accurately evaluate the prognosis of elderly patients with HCC by constructing a full age spectrum prognosis model of patients with HCC, because the clinical and pathological characteristics of young and elderly patients with HCC are different. Previous studies have shown that it is necessary to build a prognostic model to precisely assessing of the prognosis of elderly patients with HCC [19, 20]. He et al. showed that age, race, T stage, histological grade, surgery, radiotherapy, and chemotherapy were independent predictors of cancer-specific survival in elderly patients with HCC [21]. Building upon their research, we have introduced two more important indicators (AFP level and fibrosis score) to evaluate the prognosis of elderly patients with HCC. The value of AFP level and fibrosis score in evaluating the prognosis of patients with HCC has been widely verified [22,23,24]. In addition, we also enrolled patients from other center to perform external validation of the prognostic model built in this study. In conclusion, this study has constructed a more accurate and applicable prediction model for evaluating the prognosis of elderly patients with HCC.
Being older, male, and unmarried, and with bone metastasis and lung metastasis were independently associated with poor OS of HCC patients. These results were consistent with those of previous research [25, 26]. Elderly patients usually suffer from comorbidities, such as malnutrition, decreased immune function, and cognitive impairment, which limit: their treatment options and thereby results in a worse prognosis [27, 28]. The results indicated that the older the patient, the shorter their OS and the worse their prognosis. Interestingly, we found that the prognosis of unmarried patients was worse than the married did. This may be caused by the limited ability of affording the costs of continued treatment, which forces them to shorten their treatment. In addition, single patients are unable to receive the emotional support that a spouse would provide, which adversely affects their prognosis. Bone metastasis is a typical metastatic pattern in patients with HCC. Literatures reported that incidence rate of bone metastasis of HCC patients ranged from 3 to 20%, and it was on an upward trend [29, 30]. Although the management of HCC patients was improved in recent years, the prognosis of those with bone metastasis remains weak. This study found that the prognosis of HCC patients with bone metastases was worse than the ones without bone metastases (HR: 1.511; 95%CI 1.077–2.120).
Tumor size, T stage, N stage, AFP level, fibrosis score and grade were proved as independent prognostic factors of HCC patients. AFP level and tumor size have been used in many HCC prognoses models and have been proven to have good predictive ability and evaluation effects [31,32,33]. AFP is a glycoprotein synthesized from embryonic liver cells. It is the first tumor marker discovered in HCC and is widely used for the diagnosis of HCC. For example, Bai et al. found that AFP-elevated was associated with inferior survival compared with AFP-normal in patients with HCC [34]. Wu et al. indicated that the survival rate of patients with HCC decreased with an increase in tumor size. The 5-year survival rate of patients with a tumor diameter of ≤ 2 cm was 21.9%, whereas the 5-year survival rate of patients with tumor sizes of 2.1–5.0, 5.1–10.0, and 10.1–20.0 cm decreased to 14.3%, 9.2%, and 7.7%, respectively [35]. The results in this study showed that compared to those with a tumor size of < 5.6 cm, the risk of HCC patients with tumor sizes of 5.6–8.5 cm and > 8.5 cm increased by 1.186 times and 1.573 times, respectively.
Surgery and adjuvant chemotherapy can significantly improve the prognosis of patients with HCC [36, 37]. With the development of medical technology, surgery has become a standard treatment for improving the prognosis of patients with HCC. Studies have shown that HCC patients with regional lymph node infiltration or multiple metastases may benefit from surgery [38, 39]. A cohort study reported that the median overall survival (OS) of patients treated with surgery was significantly longer than that of patients treated without surgery (MOS: 20 months vs. 7 months, p < 0.001) [40]. Another study provided evidence that adjuvant chemotherapy after hepatectomy was beneficial for patients with operable HCC [41]. Oxaliplatin, sorafenib, and 5-fluorouracil are common chemotherapeutic drugs. Two randomized, placebo-controlled phase III trials demonstrated a significant improvement in OS of patients with advanced HCC [42, 43]. A recent study demonstrated that hepatic artery infusion chemotherapy combined with sorafenib can improve the OS of patients with HCC [44]. The results in this study also showed that surgery and chemotherapy can significantly improve the prognosis of patients with HCC.
To reflect the clinical value of our research, we further performed a surgical stratification analysis of age, T stage, tumor size, fibrosis score and grade. In our study, all of the elderly patients with HCC treated with surgery, had longer survival duration than non-surgical patients, which was consistent with the previous studies [45, 46]. It was shown that SR was usually a better option for older patients with HCC over the age of 74 when LT was not available. Interestingly, we found that SR and LT were a better option for elderly patients with HCC in the 0–4 FS band and 5–6 FS band, respectively. To our knowledge, this finding was reported for the first time in the SEER database. It was also shown that both in training set and internal validation, LR did not show better survival than SR does, which was consistent with other findings [47]. When surgeons were faced with the dilemma of how to choose the best treatment solution for older patients with HCC who could not undergo LT, the finding in this study may provide valuable suggestion.
Nevertheless, this study has some limitations. First, this was a retrospective study, which might result in the introduction of a partial selection bias. Second, some potential prognostic factors such as ALBI grade for liver reserve, specific chemotherapy regimens and multigene signature assessment were not included in the SEER database. In addition, we lack information on clinical indicators other than AFP level and fibrosis score. More indicators included in the study could have helped to identify clinical indicators with high specificity and sensitivity in elderly HCC patients.
Conclusion
In summary, age, sex, marital status, T stage, N stage, surgery, chemotherapy, tumor size, AFP level, fibrosis score, bone metastasis, lung metastasis, and grade were independent prognostic factors for older patients with HCC. The constructed nomogram model based on the above factors could accurately predict the prognosis of such patients. Besides, the developed online web interface of the predictive model provides easily obtained access for clinicians.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Wallace MC, Preen D, Jeffrey GP, Adams LA. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015;9(6):765–779
Zhang X, Li J, Shen F, Lau WY. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2018;33(2):347–354
Hyuna S, Jacques F, Siegel RL, Mathieu L, Isabelle S, Ahmedin J, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 Countries. Cancer J Clin. 2021;71(3):209–249
El-Serag HB, Rudolph KL. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology. 2007;132(7):2557–2576
Li C, Xu X. Biological functions and clinical applications of exosomal non-coding RNAs in hepatocellular carcinoma. Cell Mol Life Sci. 2019;76(21):4203–4219
Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154(6):1706–1718
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hep Int. 2017;11(4):317–370
Somasundaram S, Kelley RK, Venook AP. A review of hepatocellular carcinoma (HCC) staging systems. Chin Clin Oncol. 2013;2(4):33
Hung AK, Guy J. Hepatocellular carcinoma in the elderly:Meta-analysis and systematic literature review. World J Gastroenterol. 2015;21(42):12197–12210
Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765
Dingde Y, Jiamu Q, Jian W, Guoqiang L, Beicheng S, Qingxiang X. A new clinical nomogram from the TCGA database to predict the prognosis of hepatocellular carcinoma 
. Front Oncol. 2021;11:698980
Xueping W, Minjie M, Zhonglian H, Lin Z, Huilan L, Jianhua L, et al. Development and validation of a prognostic nomogram in AFP-negative hepatocellular carcinoma. Int J Biol Sci. 2019;15(1):221–228
Kena Z, Qiang Z, Congbo C. A genomic-clinicopathologic nomogram for predicting overall survival of hepatocellular carcinoma. BMC Cancer. 2020;20(1):1176
Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Overview of the surveillance, epidemiology, and end results database: evolution, data variables, and quality assurance. Curr Probl Cancer. 2012;36(4):183–190
Zhuang W, Chen J, Li Y, Liu W. Valuation of lymph node dissection in localized high-risk renal cell cancer using X-tile software. Int Urol Nephrol. 2020;52(2):253–262
Vickers AJ, Van Calster B, Steyerberg E. Decision curves, calibration, and subgroups. J Clinical Oncol. 2017;35(4):472–473
Liu K, Huang G, Chang P, Zhang W, Li T, Dai Z, et al. Construction and validation of a nomogram for predicting cancer-specific survival in hepatocellular carcinoma patients. Sci Rep. 2020;10(1):21376
Ding J, Wen Z. Survival improvement and prognosis for hepatocellular carcinoma: analysis of the SEER database. BMC Cancer. 2021;21(1):1157
Wan S, Nie Y, Zhu X. Development of a prognostic scoring model for predicting the survival of elderly patients with hepatocellular carcinoma. PeerJ. 2020;8: e8497
He T, Chen T, Liu X, Zhang B, Yue S, Cao J, et al. A web-based prediction model for cancer-specific survival of elderly patients with early hepatocellular carcinoma: a study based on SEER database. Front Public Health. 2022;9: 789026
Liu Z, Pu Y, Bao Y, He S. Investigation of potential molecular biomarkers for diagnosis and prognosis of AFP-negative HCC. Int J General Med. 2021;14:4369–4380
Luo P, Wu S, Yu Y, Ming X, Li S, Zuo X, et al. Current status and perspective biomarkers in AFP negative HCC: towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Pathol Oncol Res. 2020;26(2):599–603
Tseng TC, Choi J, Nguyen MH, Peng CY, Siakavellas S, Papatheodoridis G, et al. One-year Fibrosis-4 index helps identify minimal HCC risk in non-cirrhotic chronic hepatitis B patients with antiviral treatment. Hep Int. 2021;15(1):105–113
Wu C, Ren X, Zhang Q. Incidence, risk factors, and prognosis in patients with primary hepatocellular carcinoma and lung metastasis: a population-based study. Cancer Manag Res. 2019;11:2759–2768
Chuan H, Jiaxin Y, Zhangheng H, Chuan L, Yijun L, Yuexin T, et al. Diagnostic and prognostic nomograms for bone metastasis in hepatocellular carcinoma. BMC Cancer. 2020;20(1):494
Burdett N, Vincent AD, O’Callaghan M, Kichenadasse G. Competing risks in older patients with cancer: a systematic review of geriatric oncology trials. J Natl Cancer Inst. 2018;110(8):825–830
Lee L, Cheung WY, Atkinson E, Krzyzanowska MK. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol. 2011;29(1):106–117
Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1(3–4):144–158
Attili VS, Babu KG, Lokanatha D, Bapsy PP, Ramachandra C, Rajshekar H. Bone metastasis in hepatocellular carcinoma: need for reappraisal of treatment. J Cancer Res Ther. 2008;4(2):93–94
Tang Y, Li K, Cai Z, Xie Y, Tan X, Su C, et al. HSP90α combined with AFP and TK1 improved the diagnostic value for hepatocellular carcinoma. Biomark Med. 2020;14(10):869–878
Wang Y, Zhang C, Zhang P, Guo G, Jiang T, Zhao X, et al. Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med. 2018;7(5):1670–1679
Yang A, Xiao W, Chen D, Wei X, Huang S, Lin Y, et al. The power of tumor sizes in predicting the survival of solitary hepatocellular carcinoma patients. Cancer Med. 2018;7(12):6040–6050
Bai DS, Zhang C, Chen P, Jin SJ, Jiang GQ. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep. 2017;7(1):12870
Qiu WQ, Shi JF, Guo LW, Mao AY, Huang HY, Hu GY, et al. Medical expenditure for liver cancer in urban China: a 10-year multicenter retrospective survey (2002–2011). J Cancer Res Ther. 2018;14(1):163–170
Zhang W, Zhang B, Chen XP. Adjuvant treatment strategy after curative resection for hepatocellular carcinoma. Front Med. 2021;15(2):155–169
Adhoute X, Pénaranda G, Raoul JL, Bollon E, Pol B, Letreut YP, et al. NIACE score for hepatocellular carcinoma patients treated by surgery or transarterial chemoembolization. Eur J Gastroenterol Hepatol. 2017;29(6):706–715
Xiaohong S, Huikai L, Feng W, Ti Z, Yunlong C, Qiang L. Clinical significance of lymph node metastasis in patients undergoing partial hepatectomy for hepatocellular carcinoma. World J Surg. 2010;34(5):1028–1033
Xing L, Guo HB, Kan JL, Liu SG, Lv HT, Liu JH, et al. Clinical outcome of open surgery versus laparoscopic surgery for cirrhotic hepatocellular carcinoma patients: a meta-analysis. Eur J Gastroenterol Hepatol. 2019;32(2):239–245
Zheng Z, Liang W, Wang D, Schroder PM, Ju W, Wu L, et al. Adjuvant chemotherapy for patients with primary hepatocellular carcinoma: a meta-analysis. Int J Cancer. 2015;136(6):E751–E759
Lei C, Tao S, Shi C, Yanqiao R, Fan Y, Chuansheng Z. The efficacy of surgery in advanced hepatocellular carcinoma: a cohort study. World J Surg Oncol. 2020;18(1):119
Pugh RN. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(23):2497–2498
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34
Bin LR, Yang Z, Ke HM, Sheng WD, Yun BX, Xing HY, et al. Hepatic arterial infusion chemotherapy of oxaliplatin, fluorouracil, and leucovorin with or without sorafenib as initial treatment for advanced hepatocellular carcinoma. Front Oncol. 2021;11: 619461
Shaya FT, Breunig IM, Seal B, Mullins CD, Chirikov VV, Hanna N. Comparative and cost effectiveness of treatment modalities for hepatocellular carcinoma in SEER-medicare. Pharmacoeconomics. 2014;32(1):63–74
Kamarajah SK. Fibrosis score impacts survival following resection for hepatocellular carcinoma (HCC): a surveillance, end results and epidemiology (SEER) database analysis. Asian J Surg. 2018;41(6):551–561
Zhang QQ, Wu PY, Bahde M, Zhang LF, Zhou ZH, Liu H, et al. Do elderly patients with stage I-II hepatocellular carcinoma benefit from more radical surgeries? A population-based analysis. Front Oncol. 2020;10:479
Acknowledgements
We are very grateful to the SEER database. We would also like to thank all the participants of this project and investigators for collecting the data.
Funding
This work was supported by grants from the Natural Science Foundation of Zhejiang Province (grant number LQ21H190004) and Huzhou Municipal Bureau of Science and Technology (grant number 2020GZ43). The funding sources had no role in the study design; in the writing of the report; or in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
JT, XW, ZZ and JC designed the research. JT, XL, YY, YZ, JN, YH and XX collected and organized data. JT, HQ, ZZ and YL analyzed the data. JT, WJ, JC and XW drafted the manuscript. XW, JC and ZZ contributed to the critical revision of the manuscript. All authors contributed to the manuscript and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
Juntao Tan, Yue Yu, Xiantian Lin, Yuxin He, Wen Jin, Hong Qian, Ying Li, Xiaomei Xu, Yuxi Zhao, Jianwen Ning, Zhengyu Zhang, Jingjing Chen, and Xiaoxin Wu declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (Ethical approval No. IIT20230048B). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, J., Yu, Y., Lin, X. et al. OHCCPredictor: an online risk stratification model for predicting survival duration of older patients with hepatocellular carcinoma. Hepatol Int 18, 550–567 (2024). https://doi.org/10.1007/s12072-023-10516-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10516-x