Abstract
Background and aims
Antiviral agents for chronic hepatitis B (CHB) reduced the risk of hepatocellular carcinoma (HCC) development. The outcomes of entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF) were compared in patients with CHB.
Methods
Between 2017 and 2019, treatment-naïve patients with CHB treated with ETV, TDF, and TAF were recruited from three Korean tertiary institutes. The cumulative incidences of HCC and orthotopic liver transplantation (OLT) or mortality were calculated and compared using Kaplan–Meier analysis before and after trimatch.
Results
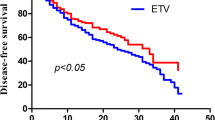
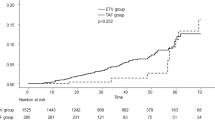
Among recruited 2082 patients, 43 patients developed HCC, whereas 66 developed OLT or mortality. Before trimatch, the cumulative incidence of HCC was statistically similar among patients treated with three antiviral agents (p = 0.340). However, the cumulative probability of OLT or mortality development in patients treated with ETV or TDF was significantly higher than that of patients with TAF before trimatch (all p < 0.05). On multivariate analysis, male sex [hazard ratio (HR) 2.990] and older age (HR 1.044) were independently associated with an increased risk of HCC development, whereas higher platelet count (HR 0.993) was independently associated with a decreased risk (all p < 0.05). The type of antiviral agents did not significantly influence the risk of HCC and OLT or mortality development (all p > 0.05). After trimatch, no significant difference in the cumulative probability for HCC and OLT or mortality according to antiviral agents was found (all p > 0.05).
Conclusions
The outcomes of ETV, TDF, and TAF on the risk of HCC and OLT or mortality were statistically similar in treatment-naïve patients with CHB.



Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CHB:
-
Chronic hepatitis B
- HCC:
-
Hepatocellular carcinoma
- ETV:
-
Entecavir
- TDF:
-
Tenofovir disoproxil fumarate
- TAF:
-
Tenofovir alafenamide
- OLT:
-
Orthotopic liver transplantation
- HR:
-
Hazard ratio
- AVT:
-
Antiviral therapy
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- HBV:
-
Hepatitis B virus
- IQR:
-
Interquartile range
- HBeAg:
-
Hepatitis B e antigen
References
Lee SW, Choi J, Kim SU, Lim YS. Entecavir versus tenofovir in patients with chronic hepatitis B: enemies or partners in the prevention of hepatocellular carcinoma. Clin Mol Hepatol. 2021;27(3):402–412. https://doi.org/10.3350/cmh.2021.0179
Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52(4):594–604. https://doi.org/10.1016/j.jhep.2009.10.033
Kim BH, Park JW. Epidemiology of liver cancer in South Korea. Clin Mol Hepatol. 2018;24(1):1–9. https://doi.org/10.3350/cmh.2017.0112
Lok AS, McMahon BJ, Brown RS Jr, Wong JB, Ahmed AT, Farah W, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology. 2016;63(1):284–306. https://doi.org/10.1002/hep.28280
Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283. https://doi.org/10.1002/hep.28156
Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. https://doi.org/10.1016/j.jhep.2017.03.021
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. https://doi.org/10.1007/s12072-015-9675-4
Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim YS. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: a Korean nationwide cohort study. JAMA Oncol. 2019;5(1):30–36. https://doi.org/10.1001/jamaoncol.2018.4070
Kim SU, Seo YS, Lee HA, Kim MN, Lee YR, Lee HW, et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naive chronic hepatitis B in South Korea. J Hepatol. 2019;71(3):456–464. https://doi.org/10.1016/j.jhep.2019.03.028
Papatheodoridis GV, Dalekos GN, Idilman R, Sypsa V, Van Boemmel F, Buti M, et al. Similar risk of hepatocellular carcinoma during long-term entecavir or tenofovir therapy in Caucasian patients with chronic hepatitis B. J Hepatol. 2020;73(5):1037–1045. https://doi.org/10.1016/j.jhep.2020.06.011
Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV infection in China. Gastroenterology. 2020;158(1):215-225.e216. https://doi.org/10.1053/j.gastro.2019.09.025
Charlton MR, Alam A, Shukla A, Dashtseren B, Lesmana CRA, Duger D, et al. An expert review on the use of tenofovir alafenamide for the treatment of chronic hepatitis B virus infection in Asia. J Gastroenterol. 2020;55(9):811–823. https://doi.org/10.1007/s00535-020-01698-4
KASL. KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2012;18(2):109–162. https://doi.org/10.3350/cmh.2012.18.2.109
KASL. KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2016;22(1):18–75. https://doi.org/10.3350/cmh.2016.22.1.18
Korean Liver Cancer Study. Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015;16(3):465–522. https://doi.org/10.3348/kjr.2015.16.3.465
Yim HJ, Kim JH, Park JY, Yoon EL, Park H, Kwon JH, et al. Comparison of clinical practice guidelines for the management of chronic hepatitis B: when to start, when to change, and when to stop. Clin Mol Hepatol. 2020;26(4):411–429. https://doi.org/10.3350/cmh.2020.0049
Hsu YC, Wong GL, Chen CH, Peng CY, Yeh ML, Cheung KS, et al. Tenofovir versus entecavir for hepatocellular carcinoma prevention in an international consortium of chronic hepatitis B. Am J Gastroenterol. 2020;115(2):271–280. https://doi.org/10.14309/ajg.0000000000000428
Kim BG, Park NH, Lee SB, Lee H, Lee BU, Park JH, et al. Mortality, liver transplantation and hepatic complications in patients with treatment-naïve chronic hepatitis B treated with entecavir vs tenofovir. J Viral Hepat. 2018;25(12):1565–1575. https://doi.org/10.1111/jvh.12971
Lee SW, Kwon JH, Lee HL, Yoo SH, Nam HC, Sung PS, et al. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naïve patients with chronic hepatitis B in Korea: a large-scale, propensity score analysis. Gut. 2020;69(7):1301–1308. https://doi.org/10.1136/gutjnl-2019-318947
Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018;68(4):672–681. https://doi.org/10.1016/j.jhep.2017.11.039
Seto WK, Asahina Y, Brown TT, Peng CY, Stanciu C, Abdurakhmanov D, et al. Improved bone safety of tenofovir alafenamide compared to tenofovir disoproxil fumarate over 2 years in patients with chronic HBV infection. Clin Gastroenterol Hepatol. 2018. https://doi.org/10.1016/j.cgh.2018.06.023
Choi H, Seo GH. Entecavir versus tenofovir for the prevention of hepatocellular carcinoma in treatment-naïve chronic hepatitis B patients in Korea. J Korean Med Sci. 2021;36(14): e89. https://doi.org/10.3346/jkms.2021.36.e89
Lee HW, Cho YY, Lee H, Lee JS, Kim SU, Park JY, et al. Impact of tenofovir alafenamide vs. entecavir on hepatocellular carcinoma risk in patients with chronic hepatitis B. Hepatol Int. 2021. https://doi.org/10.1007/s12072-021-10234-2
Su F, Berry K, Ioannou GN. No difference in hepatocellular carcinoma risk between chronic hepatitis B patients treated with entecavir versus tenofovir. Gut. 2021;70(2):370–378. https://doi.org/10.1136/gutjnl-2019-319867
Murakami E, Wang T, Park Y, Hao J, Lepist EI, Babusis D, et al. Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy. Antimicrob Agents Chemother. 2015;59(6):3563–3569. https://doi.org/10.1128/aac.00128-15
Choi J, Kim GA, Han S, Lim YS. Earlier alanine aminotransferase normalization during antiviral treatment is independently associated with lower risk of hepatocellular carcinoma in chronic hepatitis B. Am J Gastroenterol. 2020;115(3):406–414. https://doi.org/10.14309/ajg.0000000000000490
Wong GL, Chan HL, Tse YK, Yip TC, Lam KL, Lui GC, et al. Normal on-treatment ALT during antiviral treatment is associated with a lower risk of hepatic events in patients with chronic hepatitis B. J Hepatol. 2018;69(4):793–802. https://doi.org/10.1016/j.jhep.2018.05.009
Bae SH, Yoon SK, Jang JW, Kim CW, Nam SW, Choi JY, et al. Hepatitis B virus genotype C prevails among chronic carriers of the virus in Korea. J Korean Med Sci. 2005;20(5):816–820. https://doi.org/10.3346/jkms.2005.20.5.816
Funding
This study was funded in part by the grant from Bristol-Myers Squibb (Grant number: MB007-026). In addition, this study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2019R1A2C4070136). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conception: HYC, DHS, J-HL and SUK; study design: HYC, DHS, J-HL and SUK; participation in patient management and data collection: SHA, YJK, J-HY; contribution to the data acquisition: DHS and J-HL; responsibility for writing the paper: HYC, DHS, J-HL and SUK; statistical analysis: SHA, HYC and SUK. All authors reviewed the paper and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
None to declare
Ethics approval
The study protocol satisfied the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review boards of all three institutes.
Consent to participate
The requirement for written informed consent was waived because of the retrospective nature of this study.
Consent for publication
The requirement for written informed consent was waived because of the retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12072_2021_10262_MOESM1_ESM.tif
Supplementary figure 2. Complete virological response (HBV DNA < 20 IU/mL) and biochemical response (ALT ≤ 40 IU/L) according to antiviral therapy. ETV, entecavir; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide; HBV, hepatitis B virus. (TIF 573 KB)
Rights and permissions
About this article
Cite this article
Chon, H.Y., Ahn, S.H., Kim, Y.J. et al. Efficacy of entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide in treatment-naive hepatitis B patients. Hepatol Int 15, 1328–1336 (2021). https://doi.org/10.1007/s12072-021-10262-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10262-y