Abstract
Background and aims
Whether entecavir (ETV) or tenofovir alafenamide (TAF) is better at preventing hepatocellular carcinoma (HCC) development among patients with chronic hepatitis B (CHB) remains unclear. The present study was conducted to explore the ability of these two antivirals to prevent HCC.
Methods
From 2012 to 2019, treatment-naïve CHB patients undergoing ETV or TAF therapy were recruited at three academic teaching hospitals. The TAF group comprised patients starting TAF as first-line antiviral and those switching antivirals from tenofovir disoproxil fumarate to TAF. Patients with decompensated cirrhosis or HCC at enrollment were excluded from the analysis. Cumulative probabilities of HCC were assessed using the Kaplan–Meier method.
Results
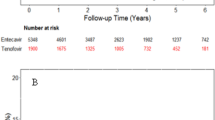
In total, 1810 patients (1525 and 285 in ETV and TAF groups, respectively) were recruited. The annual HCC incidence was statistically not different between the ETV and TAF groups (1.67 vs. 1.19 per 100 person-years, respectively) with an adjusted hazard ratio (HR) of 0.681 (p = 0.255), as determined by multivariate analysis. Male, hypertension, liver cirrhosis, FIB-4 index, and albumin were independent prognostic factors for HCC development. Propensity score-matched and inverse probability of treatment weighting analyses yielded similar results, with non-statistically different HCC incidence between the ETV and TAF groups (1.07 vs. 1.19 per 100 person-years (HR = 0.973; p = 0.953) and 1.67 vs. 1.89 per 100 person-years, respectively (HR = 0.949; p = 0.743).
Conclusions
These findings suggest that ETV- and TAF-treated CHB patients have similar risk of developing HCC. Further studies with the larger sample size and longer follow-up are needed to validate these results.



Similar content being viewed by others
Data availability
Yes.
Abbreviations
- HBV:
-
Hepatitis B virus
- CHB:
-
Chronic hepatitis B
- HCC:
-
Hepatocellular carcinoma
- NUC:
-
Nucleos(t)ide analogue
- ETV:
-
Entecavir
- TDF:
-
Tenofovir disoproxil fumarate
- TAF:
-
Tenofovir alafenamide
- HR:
-
Hazard ratio
- PS:
-
Propensity scores
- HBeAg:
-
Hepatitis B e-antigen
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- IPTW:
-
Inverse probability of treatment weighting
References
KASL. KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2019;25(2):93–159. https://doi.org/10.3350/cmh.2019.1002.
EASL. Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. https://doi.org/10.1016/j.jhep.2017.03.021.
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. https://doi.org/10.1002/hep.29800.
Yim HJ, Kim JH, Park JY, Yoon EL, Park H, Kwon JH, et al. Comparison of clinical practice guidelines for the management of chronic hepatitis B: when to start, when to change, and when to stop. Clin Mol Hepatol. 2020;26(4):411–429. https://doi.org/10.3350/cmh.2020.0049.
Park SH, Plank LD, Suk KT, Park YE, Lee J, Choi JH, et al. Trends in the prevalence of chronic liver disease in the Korean adult population, 1998–2017. Clin Mol Hepatol. 2020;26(2):209–215. https://doi.org/10.3350/cmh.2019.0065.
Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130(3):678–686. https://doi.org/10.1053/j.gastro.2005.11.016.
Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73. https://doi.org/10.1001/jama.295.1.65.
Schwabl P, Bota S, Salzl P, Mandorfer M, Payer BA, Ferlitsch A, et al. New reliability criteria for transient elastography increase the number of accurate measurements for screening of cirrhosis and portal hypertension. Liver Int. 2015;35(2):381–390. https://doi.org/10.1111/liv.12623.
Kim MN, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, et al. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology. 2015;61(6):1851–1859. https://doi.org/10.1002/hep.27735.
Con D, Goodwin T, Majeed A, Roberts S, Kemp W. Comparison of 48-week efficacy of tenofovir vs entecavir for patients with chronic hepatitis B: a network meta-analysis. J Viral Hepat. 2021;28(1):40–50. https://doi.org/10.1111/jvh.13400.
Lampertico P, Buti M, Fung S, Ahn SH, Chuang WL, Tak WY, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in virologically suppressed patients with chronic hepatitis B: a randomised, double-blind, phase 3, multicentre non-inferiority study. Lancet Gastroenterol Hepatol. 2020;5(5):441–453. https://doi.org/10.1016/s2468-1253(19)30421-2.
Yim SY, Um SH, Jung JY, Seo YS, Yim HJ, Ryu HS, et al. Role of hepatitis B surface antigen (HBsAg) in identifying true inactive HBsAg carriers infected with genotype C hepatitis B virus. J Clin Gastroenterol. 2014;48(2):166–171. https://doi.org/10.1097/MCG.0b013e3182a4711d.
Liu J, Yang HI, Lee MH, Jen CL, Batrla-Utermann R, Lu SN, et al. Serum levels of hepatitis B surface antigen and DNA can predict inactive carriers with low risk of disease progression. Hepatology. 2016;64(2):381–389. https://doi.org/10.1002/hep.28552.
Choi J, Han S, Kim N, Lim YS. Increasing burden of liver cancer despite extensive use of antiviral agents in a hepatitis B virus-endemic population. Hepatology. 2017;66(5):1454–1463. https://doi.org/10.1002/hep.29321.
Ha Y, Chon YE, Kim MN, Lee JH, Hwang SG. Hepatocellular carcinoma and death and transplantation in chronic hepatitis B treated with entecavir or tenofovir disoproxil fumarate. Sci Rep. 2020;10(1):13537. https://doi.org/10.1038/s41598-020-70433-z.
Kim SU, Seo YS, Lee HA, Kim MN, Lee YR, Lee HW, et al. A multicenter study of entecavir vs tenofovir on prognosis of treatment-naïve chronic hepatitis B in South Korea. J Hepatol. 2019;71(3):456–464. https://doi.org/10.1016/j.jhep.2019.03.028.
Papatheodoridis GV, Dalekos GN, Idilman R, Sypsa V, Van Boemmel F, Buti M, et al. Similar risk of hepatocellular carcinoma during long-term entecavir or tenofovir therapy in Caucasian patients with chronic hepatitis B. J Hepatol. 2020;73(5):1037–1045. https://doi.org/10.1016/j.jhep.2020.06.011.
Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV infection in China. Gastroenterology. 2020;158(1):215–225. https://doi.org/10.1053/j.gastro.2019.09.025.
Cheung KS, Mak LY, Liu SH, Cheng HM, Seto WK, Yuen MF, et al. Entecavir vs tenofovir in hepatocellular carcinoma prevention in chronic hepatitis B infection: a systematic review and meta-analysis. Clin Transl Gastroenterol. 2020;11(10):e00236. https://doi.org/10.14309/ctg.0000000000000236.
Lee SW, Kwon JH, Lee HL, Yoo SH, Nam HC, Sung PS, et al. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naïve patients with chronic hepatitis B in Korea: a large-scale, propensity score analysis. Gut. 2020;69(7):1301–1308. https://doi.org/10.1136/gutjnl-2019-318947.
Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):196–206. https://doi.org/10.1016/s2468-1253(16)30107-8.
Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):185–195. https://doi.org/10.1016/s2468-1253(16)30024-3.
Lim YS, Chan HL, Seto WK, Ning Q, Agarwal K, Janssen HLA, et al. Impact of treatment with tenofovir alafenamide or tenofovir disoproxil fumarate on hepatocellular carcinoma incidence in patients with chronic hepatitis B. Hepatology. 2019;70(S1):126A.
Esfeh JM, Hajifathalian K, Ansari-Gilani K. Sensitivity of ultrasound in detecting hepatocellular carcinoma in obese patients compared to explant pathology as the gold standard. Clin Mol Hepatol. 2020;26(1):54–59. https://doi.org/10.3350/cmh.2019.0039.
Maruyama H, Kato N. Advances in ultrasound diagnosis in chronic liver diseases. Clin Mol Hepatol. 2019;25(2):160–167. https://doi.org/10.3350/cmh.2018.1013.
Yang JD. Detect or not to detect very early stage hepatocellular carcinoma? The western perspective. Clin Mol Hepatol. 2019;25(4):335–343. https://doi.org/10.3350/cmh.2019.0010.
EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019.
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. https://doi.org/10.1002/hep.29086.
Lee S, Kim SS, Chang DR, Kim H, Kim MJ. Comparison of LI-RADS 2018 and KLCA-NCC 2018 for noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. Clin Mol Hepatol. 2020;26(3):340–351. https://doi.org/10.3350/cmh.2020.0004.
Kim YY, Park MS, Aljoqiman KS, Choi JY, Kim MJ. Gadoxetic acid-enhanced magnetic resonance imaging: hepatocellular carcinoma and mimickers. Clin Mol Hepatol. 2019;25(3):223–233. https://doi.org/10.3350/cmh.2018.0107.
Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim YS. Risk of Hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: a Korean Nationwide Cohort Study. JAMA Oncol. 2019;5(1):30–36. https://doi.org/10.1001/jamaoncol.2018.4070.
Ha I, Chung JW, Jang ES, Jeong SH, Kim JW. Comparison of the on-treatment risks for hepatocellular carcinoma between entecavir and tenofovir: a propensity score matching analysis. J Gastroenterol Hepatol. 2020;35(10):1774–1781. https://doi.org/10.1111/jgh.15031.
Choi H, Seo GH. Entecavir versus tenofovir for the prevention of hepatocellular carcinoma in treatment-naïve chronic hepatitis B patients in Korea. J Korean Med Sci. 2021;36(14): e89. https://doi.org/10.3346/jkms.2021.36.e89.
Choi J, Kim GA, Han S, Lim YS. Earlier alanine aminotransferase normalization during antiviral treatment is independently associated with lower risk of hepatocellular carcinoma in chronic hepatitis B. Am J Gastroenterol. 2020;115(3):406–414. https://doi.org/10.14309/ajg.0000000000000490.
Wong GL, Chan HL, Tse YK, Yip TC, Lam KL, Lui GC, et al. Normal on-treatment ALT during antiviral treatment is associated with a lower risk of hepatic events in patients with chronic hepatitis B. J Hepatol. 2018;69(4):793–802. https://doi.org/10.1016/j.jhep.2018.05.009.
Kim EJ, Yeon JE, Kwon OS, Lee HN, Shin SK, Kang SH, et al. Rapid alanine aminotransferase normalization with entecavir and hepatocellular carcinoma in hepatitis B virus-associated cirrhosis. Dig Dis Sci. 2017;62(3):808–816. https://doi.org/10.1007/s10620-016-4431-8.
Laccetti M, Manes G, Uomo G, Lioniello M, Rabitti PG, Balzano A. Flumazenil in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double blind randomized placebo controlled study. Dig Liver Dis. 2000;32(4):335–338. https://doi.org/10.1016/s1590-8658(00)80027-4.
Chao X, Qian H, Wang S, Fulte S, Ding WX. Autophagy and liver cancer. Clin Mol Hepatol. 2020;26(4):606–617. https://doi.org/10.3350/cmh.2020.0169.
Yoon SM, Kim SY, Lim YS, Kim KM, Shim JH, Lee D, et al. Stereotactic body radiation therapy for small (≤5 cm) hepatocellular carcinoma not amenable to curative treatment: results of a single-arm, phase II clinical trial. Clin Mol Hepatol. 2020;26(4):506–515. https://doi.org/10.3350/cmh.2020.0038.
Kim BG, Park NH, Lee SB, Jeon S, Park JH, Jung SW, et al. The risk of hepatocellular carcinoma within and beyond the first 5 years of entecavir in Korean patients with chronic hepatitis B. Liver Int. 2018. https://doi.org/10.1111/liv.13938.
Murata K, Asano M, Matsumoto A, Sugiyama M, Nishida N, Tanaka E, et al. Induction of IFN-λ3 as an additional effect of nucleotide, not nucleoside, analogues: a new potential target for HBV infection. Gut. 2018;67(2):362–371. https://doi.org/10.1136/gutjnl-2016-312653.
Sinn DH, Lee J, Goo J, Kim K, Gwak GY, Paik YH, et al. Hepatocellular carcinoma risk in chronic hepatitis B virus-infected compensated cirrhosis patients with low viral load. Hepatology. 2015;62(3):694–701. https://doi.org/10.1002/hep.27889.
Cho YY, Lee JH, Chang Y, Nam JY, Cho H, Lee DH, et al. Comparison of overall survival between antiviral-induced viral suppression and inactive phase chronic hepatitis B patients. J Viral Hepat. 2018;25(10):1161–1171. https://doi.org/10.1111/jvh.12927.
Lee SB, Jeong J, Park JH, Jung SW, Jeong ID, Bang SJ, et al. Low-level viremia and cirrhotic complications in patients with chronic hepatitis B according to adherence to entecavir. Clin Mol Hepatol. 2020;26(3):364–375. https://doi.org/10.3350/cmh.2020.0012.
Hsu YC, Yip TC, Ho HJ, Wong VW, Huang YT, El-Serag HB, et al. Development of a scoring system to predict hepatocellular carcinoma in Asians on antivirals for chronic hepatitis B. J Hepatol. 2018;69(2):278–285. https://doi.org/10.1016/j.jhep.2018.02.032.
Lam L, Fontaine H, Bourliere M, Lusivika-Nzinga C, Dorival C, Thabut D, et al. Predictive factors for hepatocellular carcinoma in chronic hepatitis B using structural equation modeling: a prospective cohort study. Clin Res Hepatol Gastroenterol. 2021:101713. https://doi.org/10.1016/j.clinre.2021.101713.
Kim BK, Revill PA, Ahn SH. HBV genotypes: relevance to natural history, pathogenesis and treatment of chronic hepatitis B. Antivir Ther. 2011;16(8):1169–1186. https://doi.org/10.3851/imp1982.
Lee HW, Kim EH, Lee J, Kim SU, Park JY, Kim DY, et al. Natural history of untreated HBeAg-positive chronic HBV infection with persistently elevated HBV DNA but normal alanine aminotransferase. Clin Transl Gastroenterol. 2020;11(3): e00140. https://doi.org/10.14309/ctg.0000000000000140.
Kim SU, Seo YS, Lee HA, Kim MN, Lee EJ, Shin HJ, et al. Hepatocellular carcinoma risk steadily persists over time despite long-term antiviral therapy for hepatitis B: a multicenter study. Cancer Epidemiol Biomarkers Prev. 2020;29(4):832–837. https://doi.org/10.1158/1055-9965.Epi-19-0614.
Lee HW, Kim SU, Park JY, Baatarkhuu O, Kim DY, Ahn SH, et al. Prognosis of untreated minimally active chronic hepatitis B patients in comparison with virological responders by antivirals. Clin Transl Gastroenterol. 2019;10(6): e00036. https://doi.org/10.14309/ctg.0000000000000036.
Liu S, Zhou B, Valdes JD, Sun J, Guo H. Serum HBV RNA: a new potential biomarker for chronic hepatitis B virus infection. Hepatology. 2019;69(4):1816–1827. https://doi.org/10.1002/hep.30325.
Inoue T, Tanaka Y. Novel biomarkers for the management of chronic hepatitis B. Clin Mol Hepatol. 2020;26(3):261–279. https://doi.org/10.3350/cmh.2020.0032.
Lall S, Agarwala P, Kumar G, Sharma MK, Gupta E. The dilemma of differentiating between acute hepatitis B and chronic hepatitis B with acute exacerbation: is quantitative serology the answer? Clin Mol Hepatol. 2020;26(2):187–195. https://doi.org/10.3350/cmh.2019.0060.
Mak LY, Cloherty G, Wong DK, Gersch J, Seto WK, Fung J, et al. HBV RNA profiles in chronic hepatitis B patients under different disease phases and anti-viral therapy. Hepatology. 2020. https://doi.org/10.1002/hep.31616.
Tseng TC, Liu CJ, Hsu CY, Hong CM, Su TH, Yang WT, et al. High level of hepatitis B core-related antigen associated with increased risk of hepatocellular carcinoma in patients with chronic HBV infection of intermediate viral load. Gastroenterology. 2019;157(6):1518-1529.e1513. https://doi.org/10.1053/j.gastro.2019.08.028.
Coffin CS, Zhou K, Terrault NA. New and old biomarkers for diagnosis and management of chronic hepatitis B virus infection. Gastroenterology. 2019;156(2):355–368. https://doi.org/10.1053/j.gastro.2018.11.037.
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. https://doi.org/10.1007/s12072-017-9799-9.
Funding
This study was supported by a faculty research grant of Yonsei University College of Medicine for (6-2020-0130).
Author information
Authors and Affiliations
Contributions
HWL, HL, and BKK conducted statistical analyses, and all authors interpreted the findings. HWL, YYC, BKK, and SYP drafted the manuscript. HL, JSL, SUK, JYP, DYK, and SHA critically reviewed the manuscript for key intellectual content. All authors approved the final manuscript. BKK and SYP are the guarantors, and as such, had full access to the data and take responsibility for its integrity and accuracy.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study protocol was consistent with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of each participating institution.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Lee, H.W., Cho, Y.Y., Lee, H. et al. Impact of tenofovir alafenamide vs. entecavir on hepatocellular carcinoma risk in patients with chronic hepatitis B. Hepatol Int 15, 1083–1092 (2021). https://doi.org/10.1007/s12072-021-10234-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10234-2