Abstract
Background
Recent studies have suggested that several types of toxic bile acids (BAs) are involved in the pathogenesis of non-alcoholic steatohepatitis (NASH). In the present study, we aimed to determine whether elobixibat, an ileal bile acid transporter (IBAT) inhibitor, would ameliorate NASH in mice.
Methods
C57BL/6N mice were fed a methionine and choline-deficient (MCD) to induce NASH or standard diet as control for 8 weeks (n = 5 per group). The MCD diet-fed mice were administered elobixibat 5 days a week for 4 weeks by gavage (n = 5). The effects of the treatments on liver histopathology, proinflammatory cytokine concentrations, intestinal epithelial tight junctions, and the intestinal microbial composition were then assessed.
Results
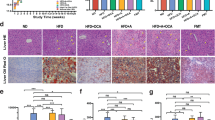
In MCD-fed mice, hepatic fibrosis and inflammatory cell infiltration developed, and the serum aspartate transaminase activity and BA concentration were higher than the control. In addition, the proinflammatory cytokine concentrations were high in the liver and mesenteric lymph nodes (MLN), and the expression of intestinal epithelium tight junction proteins, claudin1, was increased. In the intestinal microbial composition, the abundance of the Lachnospiraceae and Ruminococcaeae were decreased, whereas that of the Enterobacteriaceae was increased. Treatment with elobixibat reduced the serum BA and increased the fecal BA concentration, and ameliorated the liver inflammation and fibrosis. It also reduced the expression of proinflammatory cytokines in the liver and MLNs, and transforming growth factor-β expression in the liver. Finally, elobixibat normalized intestinal tight junction protein level and the composition of the intestinal microbiota.
Conclusion
Elobixibat ameliorates NASH-related histopathology, reduces cytokine expression, and normalizes the intestinal microbial composition in MCD-fed mice, which suggests that it may represent a promising candidate for the therapy of NASH.






Similar content being viewed by others
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- BAs:
-
Bile acids
- IBAT:
-
Ileal bile acid transporter
- HFD:
-
High-fat diet
- LDL:
-
Low-density lipoprotein
- TJ:
-
Tight junction
- MCD:
-
Methionine and choline deficient
- AST:
-
Aspartate transaminase
- HE:
-
Hematoxylin and eosin
- NAS:
-
NAFLD activity score
- TBS:
-
Tris-buffered saline
- MLN:
-
Mesenteric lymph node
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interleukin-6
- TGF-β:
-
Transforming growth factor-β
- RT:
-
Room temperature
- RDP:
-
Ribosomal database project
- HCC:
-
Hepatocellular carcinoma
- HSCs:
-
Hepatic stellate cells
- ROS:
-
Reactive oxygen species
References
Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM 2010;103:71–83
Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J 2017;377:2063–2072
Suga T, Yamaguchi H, Ogura J, Shoji S, Maekawa M, Mano N. Altered bile acid composition and disposition in a mouse model of non-alcoholic steatohepatitis. Toxicol Appl Pharmacol 2019;379:114664
George C, Ronak P, Sandeep K, Sanjaya KS. Hepatocellular carcinoma in non-alcoholic steatohepatitis: current knowledge and implications for management. World J Hepatol 2017;9(11):533–543
Chiang JY. Bile acid metabolism and signaling. Compr Physiol 2013;3(3):1191–1212
Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, et al. The presence and severity of nonalcoholic steatosis associated with specific changes I circulating bile acids. Hepatology 2018;67:534–548
Kalhan SC, Guo L, Edmison J, Dasarathy S, McCllough AJ, Hanson RW, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metab Clin Exp 2011;60:404–413
Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 2017;65(1):350–362
Meithke AG, Zhang W, Simmons J, Taylor AE, Shi T, Shanmukhappa SK, et al. Pharmacological inhibition of apical sodium dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology 2016;63:512–523
Rao A, Kosters A, Mells JE, Zhang W, Setchell KD, Amanso AM, et al. Inhibitor of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet fed mice. Sci Trasl Med 2016;8:357ra122
Bhant BG, Rapp SR, Beaudty JA, Napawan N, Butteiger DN, Hall KA, et al. Inhibitition of ileal bile acid transport and reduced atherosclerosis in apoE-/- mice by SC435. Lipid Res 2003;44:1614–1621
Al-Dury S, Marschall HU. Ileal bile acid transporter inhibition for the treatment of chronic constipation, cholestatic pruritus, and NASH. Front Pharmacol 2018;9:931
Duarte SMB, Stefano JT, Oliveira CP. Microbiota and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH). Ann Hepatol 2019;18:416–421
Brun P, Castagliuolo I, Leo DV, Buda A, Pinzani M, Palu G, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol 2007;292:G518–G525
Qiu Z, Sheridan BS. Isolating lymphocytes from the mouse small intestinal immune system. J Vis Exp 2018;132:57281
Gallo-Oller G, Ordoñez R, Dotor J. A new background subtraction method for Western blot densitometry band quantification through image analysis software. J Immunol Methods 2018;457:1–5
Fickert P, Wagner M. Biliary bile acids in hepatobiliary injury—What is the link? J Hepatol 2017;67:619–631
Molinaro A, Wahlstrom A, Marschall HU. Role of bile acids in metabolic control. Trends Endocrinol Metab 2018;29:31–41
Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 2012;56:118–129
Yamada S, Takashima Y, Watanabe M, Nagamine R, Saito Y, Kamada N, et al. Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice. Oncotarget 2018;11:9925–9939
Svenja S, Jan B, et al. Altered microbiota diversity and bile acid signaling in cirrhotic and noncirrhotic NASH-HCC. Clin Transl Gastroenterol 2020;11:e00131
Saga K, Iwashita Y, Hidano S, Aso Y, Isaka K, Kido Y, et al. Secondary unconjugated bile acids induce hepatic stellate cell activation. Int J Mol Sci 2018;19:3043
Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem 2010;285:12037–12046
Weber CR, Nalle SC, Tretiakova M, Rubin DT, Tumer JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest 2008;11:10–120
Poritz LS, HarrisKelly LRAA, Koltun WA. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig Dis Sci 2011;56:2802–2809
Han X, Fink MP, Yang R, Delude RL. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock 2004;21:261–270
Garcia-Hernandez V, Quiros M, Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann NY Acad Sci 2017;1397:66–79
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031
Liu H, Wang J, He T, Becker S, Zhang G, Li D, et al. Butyrate: a double-edged sword for health? Adv Nutr 2018;2:21–29
Kim HN, Joo EJ, Cheong HS, Kim Y, Kim HL, Shin H, Chang Y, Ryu S. Gut microbiota and risk of persistent nonalcoholic fatty liver disease. J Clin Med 2019;8(8):1089
Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol 2017;10(1):18–26
Misawa N, Higurashi T, Takatsu T. The benefit of elobixibat in chronic constipation is associated with faecal deoxycholic acid but not effects of altered microbiota. Aliment Pharmacol Ther 2020;52(5):821–828
Sydor S, Best J. Altered microbiota diversity and bile acid signaling in cirrhotic and noncirrhotic NASH-HCC. Clin Transl Gastroenterol 2020;11(3):e00131
Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011;141(5):1773–1181
Graffner H, Gillberg PG, Rikner L, Marschall HU. The ileal bile acid transporter inhibitor A4250 decreases serum bile acids by interrupting the enterohepatic circulation. Aliment Pharmacol Ther 2016;43(2):303–310
Donnelly KI, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted with lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351
Acknowledgements
We thank Mark Cleasby, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted and ethical approval was obtained from The Animal Care and Use Committee of Fukuoka University (Permit number: 2090). This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamauchi, R., Takedatsu, H., Yokoyama, K. et al. Elobixibat, an ileal bile acid transporter inhibitor, ameliorates non-alcoholic steatohepatitis in mice. Hepatol Int 15, 392–404 (2021). https://doi.org/10.1007/s12072-020-10107-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-020-10107-0