Abstract
Purpose
Hepatocellular carcinoma (HCC) represents a major health challenge because of its increasing morbidity and mortality. The establishment of useful models of HCC can significantly contribute to unveiling its pathophysiology. We developed a novel mouse model of HCC based on type 1 diabetes and reported its histopathological features.
Methods
Newborn male ddY, Institute for Animal Reproduction (DIAR) mice were divided into two groups on the basis of streptozotocin treatment, which induces type 1 diabetes. Streptozotocin was subcutaneously injected (60 mg/g) into the treated group (DIAR-nSTZ mice), whereas physiologic solution was injected into the control group (DIAR-control mice) at 1.5 days after birth. All mice were fed a normal diet and histopathologically assessed at 6, 8, 10, 12, 19, and 27 weeks of age.
Results
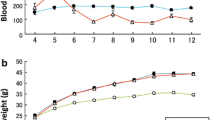
At 8 weeks, small hepatocytic nodules with mild to moderate cellular atypia were observed in the livers of DIAR-nSTZ mice, which progressed to large hepatocytic nodules with cellular atypia and infiltrating growth at 12 weeks, identical to those in well-differentiated human HCC. At 19 and 27 weeks, moderately differentiated HCC was observed in all DIAR-nSTZ mice. Conversely, no neoplastic findings were evident in DIAR-control mice. No steatosis or fibrosis was evident in either group. Immunohistochemical analysis revealed that all nodules observed in DIAR-nSTZ mice were positive for glutamine synthetase.
Conclusions
In DIAR-nSTZ mice, the development of HCC with similarity to human HCC and high reproducibility can be achieved using a short and simple protocol. We believe that this model will be useful for studying liver carcinogenesis.







Similar content being viewed by others
References
Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;47:S2–S6
Trevisani F, Cantarini MC, Wands JR, Bernardi M. Recent advances in the natural history of hepatocellular carcinoma. Carcinogenesis 2008;29:1299–1305
Loomba R, Yang HI, Su J, Brenner D, Barrett-Connor E, Iloeje U, et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol 2013;177:333–342
Deuffic S, Poynard T, Buffat L, Valleron AJ. Trends in primary liver cancer. Lancet 1998;351:214–215
Farber E. The multistep nature of cancer development. Cancer Res 1984;44:4217–4223
Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol 2009;90:367–386
Frey S, Buchmann A, Bursch W, Schulte-Hermann R, Schwarz M. Suppression of apoptosis in C3H mouse liver tumors by activated Ha-ras oncogene. Carcinogenesis 2000;21:161–166
Park TJ, Kim JY, Oh SP, Kang SY, Kim BW, Wang HJ, et al. TIS21 negatively regulates hepatocarcinogenesis by disruption of cyclin B1-Forkhead box M1 regulation loop. Hepatology 2008;47:1533–1543
Teoh NC, Dan YY, Swisshelm K, Lehman S, Wright JH, Haque J, et al. Defective DNA strand break repair causes chromosomal instability and accelerates liver carcinogenesis in mice. Hepatology 2008;47:2078–2088
Zimmers TA, Jin X, Gutierrez JC, Acosta C, McKillop IH, Pierce RH, et al. Effect of in vivo loss of GDF-15 on hepatocellular carcinogenesis. J Cancer Res Clin Oncol 2008;134:753–759
Shiota G, Harada K, Ishida M, Tomie Y, Okubo M, Katayama S, et al. Inhibition of hepatocellular carcinoma by glycyrrhizin in diethylnitrosamine-treated mice. Carcinogenesis 1990;20:59–63
Chen B, Liu L, Castonguay A, Maronpot RR, Anderson MW, You M. Dose-dependent ras mutation spectra in N-nitrosodiethylamine induced mouse liver tumors and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced mouse lung tumors. Carcinogenesis 1993;14:1603–1608
Hacker HJ, Mtiro H, Bannasch P, Vesselinovitch SD. Histochemical profile of mouse hepatocellular adenomas and carcinomas induced by a single dose of diethylnitrosamine. Cancer Res 1991;51:1952–1958
Hays T, Rusyn I, Burns AM, Kennett MJ, Ward JM, Gonzalez FJ, et al. Role of peroxisome proliferator-activated receptor-alpha (PPARalpha) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis 2005;26:219–227
Reddy JK, Rao S, Moody DE. Hepatocellular carcinomas in acatalasemic mice treated with nafenopin, a hypolipidemic peroxisome proliferator. Cancer Res 1976;36:1211–1217
Takashima K, Ito Y, Gonzalez FJ, Nakajima T. Different mechanisms of DEHP-induced hepatocellular adenoma tumorigenesis in wild-type and Ppar alpha-null mice. J Occup Health 2008;50:169–180
McGlynn KA, Hunter K, LeVoyer T, Roush J, Wise P, Michielli RA, et al. Susceptibility to aflatoxin B1-related primary hepatocellular carcinoma in mice and humans. Cancer Res 2003;63:4594–4601
Ghebranious N, Sell S. The mouse equivalent of the human p53ser249 mutation p53ser246 enhances aflatoxin hepatocarcinogenesis in hepatitis B surface antigen transgenic and p53 heterozygous null mice. Hepatology 1998;27:967–973
Salguero Palacios R, Roderfeld M, Hemmann S, Rath T, Atanasova S, Tschuschner A, et al. Activation of hepatic stellate cells is associated with cytokine expression in thioacetamide-induced hepatic fibrosis in mice. Lab Invest 2008;88:1192–1203
de Lima VM, Oliveira CP, Alves VA, Chammas MC, Oliveira EP, Stefano JT, et al. A rodent model of NASH with cirrhosis, oval cell proliferation and hepatocellular carcinoma. J Hepatol 2008;49:1055–1061
Knight B, Yeoh GC, Husk KL, Ly T, Abraham LJ, Yu C, et al. Impaired preneoplastic changes and liver tumor formation in tumor necrosis factor receptor type 1 knockout mice. J Exp Med 2000;192:1809–1818
Liquori GE, Calamita G, Cascella D, Mastrodonato M, Portincasa P, Ferri D. An innovative methodology for the automated morphometric and quantitative estimation of liver steatosis. Histol Histopathol 2009;24:49–60
Nishida T, Tsuneyama K, Fujimoto M, Nomoto K, Hayashi S, Miwa S, et al. Spontaneous onset of nonalcoholic steatohepatitis and hepatocellular carcinoma in a mouse model of metabolic syndrome. Lab Invest 2013;93:230–241
Li Z, Karlsson FA, Sandler S. Islet loss and alpha cell expansion in type 1 diabetes induced by multiple low-dose streptozotocin administration in mice. J Endocrinol 2000;165:93–99
Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer; 2010. p. 205–216
Di Tommaso L, Destro A, Seok JY, Balladore E, Terracciano L, Sangiovanni A, et al. The application of markers (HSP70 GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J Hepatol 2009;50:746–754
Christa L, Simon MT, Flinois JP, Gebhardt R, Brechot C, Lasserre C. Overexpression of glutamine synthetase in human primary liver cancer. Gastroenterology 1994;106:1312–1320
Chuma M, Sakamoto M, Yamazaki K, Ohta T, Ohki M, Asaka M, et al. Expression profiling in multistage hepatocarcinogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology 2003;37:198–207
Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003;125:89–97
Kaufmann O, Dietel M. Thyroid transcription factor-1 is the superior immunohistochemical marker for pulmonary adenocarcinomas and large cell carcinomas compared to surfactant proteins A and B. Histopathology 2000;36:8–16
Wu KK, Huan Y. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 2008;40:1–14
Lauder I, Abascal J, Cartwright RA, Farndon JR, Johnston ID. Liver tumours following streptozotocin administration in rats and the effects of pancreatic islet cell transplantation. Carcinogenesis 1981;2:799–803. doi:10.1093/carcin/2.8.799
Bell RH Jr, Hye RJ, Miyai K. Streptozotocin-induced liver tumors in the Syrian hamster Carcinogenesis 1984;5:1235–1238
De Minicis S, Kisseleva T, Francis H, Baroni GS, Benedetti A, Brenner D, et al. Liver carcinogenesis: rodent models of hepatocarcinoma and cholangiocarcinoma. Dig Liver Dis 2013;45:450–459
Farber E, Solt D, Cameron R, Laishes B, Ogawa K, Medline A. Newer insights into the pathogenesis of liver cancer. Am J Pathol 1977;89:477–482
Klinman NR, Erslev AJ. Cellular response to partial hepatectomy. Proc Soc Exp Biol Med 1963;112:338–340
Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol 1991;22:172–178
International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 2009;49:658–664
Fujii M, Shibazaki Y, Wakamatsu K, Honda Y, Kawauchi Y, Suzuki K, et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol Morphol 2013;46:141–152
Dombrowski F, Mathieu C, Evert M. Hepatocellular neoplasms induced by low-number pancreatic islet transplants in autoimmune diabetic BB/Pfd rats. Cancer Res 2006;66:1833–1843
Acknowledgements
We thank Tokimasa Kumada, Mitsuko Sutoh, and Emu Oda for their help and technical assistance with our experiments. We would also like to thank Yukari Inoue for her excellent support during the preparation of this manuscript. This work was supported by JSPS KAKENHI grant nos. 0293341 and 10293341.
Compliance with ethical requirements and Conflict of interest
Animal care and surgical procedures were approved by the Institute of Animal Reproduction in accordance with criteria outlined in the “Principles of laboratory animal care” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication no. 85-23 revised 1985). Hayato Baba, Koichi Tsuneyama, Takeshi Nishida, Hideki Hatta, Takahiko Nakajima, Kazuhiro Nomoto, Shinichi Hayashi, Shigeharu Miwa, Yuko Nakanishi, Ryoji Hokao, and Johji Imura1 declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baba, H., Tsuneyama, K., Nishida, T. et al. Neonatal streptozotocin treatment causes type 1 diabetes and subsequent hepatocellular carcinoma in DIAR mice fed a normal diet. Hepatol Int 8, 415–424 (2014). https://doi.org/10.1007/s12072-014-9541-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-014-9541-9