Abstract
Background
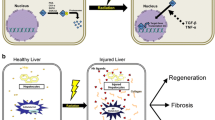
It is unclear why the response to radiation in the female liver is different from that of the male liver. Hedgehog (Hh) that remains latent in healthy adult livers is activated in the injured liver and promotes the proliferation of progenitors and myofibroblastic hepatic stellate cells, leading to hepatic fibrosis.
Objective
These findings have led to the hypothesis that the gender-specific expression of Hh signaling could affect the different response of the female liver to radiation.
Methods
Male and female mice irradiated with a single dose of 6 Gy were killed at 1 week post irradiation, and the livers were collected for biochemical analysis.
Results
A greater accumulation of fatty hepatocytes and apoptotic cells was observed in irradiated female mice. Sox-9 and pancytokeratin-positive cells were expanded in the livers of irradiated female, but not male, mice. The expression of the Hh ligand, Sonic Hh, Hh receptor, Smoothened, and Hh-target gene, Gli2, showed a greater increase in the liver of radiation-treated female. The levels of epithelial-to-mesenchymal transition (EMT)-stimulating factor, transforming growth factor-β, collagen α1, and N-cadherin were upregulated, while the EMT inhibitor, bmp7, was downregulated in the damaged liver of females compared to controls. In addition, increased fibrosis was seen in the injured livers of female mice. No significant changes in Hh expression and EMT were detected in the irradiated male mice.
Conclusion
These results demonstrated that the increased expression of Hh signaling contributed to the different repair process in the irradiated female mice by promoting proliferation of progenitor and EMT process.





Similar content being viewed by others
Abbreviations
- EMT:
-
Epithelial-to-mesenchymal transition
- Gli:
-
Glioblastoma
- Hh:
-
Hedgehog
- HIP:
-
Hedgehog-interacting protein
- H&E:
-
Hematoxylin and eosin
- HSC:
-
Hepatic stellate cell
- Ihh:
-
Indian hedgehog
- IHC:
-
Immunohistochemistry
- MF-HSC:
-
Myofibroblastic hepatic stellate cells
- Pan-CK:
-
Pancytokeratin
- Ptc:
-
Patched
- Shh:
-
Sonic hedgehog
- Smo:
-
Smoothened
- TGF-β:
-
Transforming growth factor-β
References
Delaney G, Barton M, Jacob S. Estimation of an optimal radiotherapy utilization rate for melanoma. Cancer. 2004;100:1293–1301
Henderson MA, Valluri S, DesRosiers C, Lopez JT, Batuello CN, Caperell-Grant A, et al. Effect of gender on radiation-induced cataractogenesis. Radiat Res. 2009;172:129–133
Inal ME, Akgün A, Kahraman A. Radioprotective effects of exogenous glutathione against whole-body γ-ray irradiation: age- and gender-related changes in malondialdehyde levels, superoxide dismutase and catalase activities in rat liver. Methods Find Exp Clin Pharmacol. 2002;24:209–212
Sorensen KJ, Zetterberg LA, Nelson DO, Grawe J, Tucker JD. The in vivo dose rate effect of chronic gamma radiation in mice: translocation and micronucleus analyses. Mutat Res/Fundam Mol Mech Mutagenesis. 2000;457:125–136
Jung Y, Witek RP, Syn WK, Choi SS, Omenetti A, Premont R, et al. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–665
Omenetti A, Choi S, Michelotti G, Diehl AM. Hedgehog signaling in the liver. J Hepatol. 2011;54:366–373
Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cell Signal. 2009;21:1023–1034
Sicklick JK, Li YX, Choi SS, Qi Y, Chen W, Bustamante M, et al. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest. 2005;85:1368–1380
Li N, Zhang L, Li H, Fang B, Administration of Granulocyte Colony-Stimulating Factor Ameliorates Radiation-Induced Hepatic Fibrosis in Mice. Transplant Proc. Elsevier, 2010, pp. 3833-3839.
Jaal J, Dörr W. Radiation-induced damage to mouse urothelial barrier. Radiother Oncol. 2006;80:250–256
Jung Y, Oh S-H, Zheng D, Shupe TD, Witek RP, Petersen BE. A potential role of somatostatin and its receptor SSTR4 in the migration of hepatic oval cells. Lab Invest. 2006;86:477–489
Tolosa L, Bonora-Centelles A, Teresa Donato M, Pareja E, Negro A, López S, et al. Steatotic liver: a suitable source for the isolation of hepatic progenitor cells. Liver Int. 2011;31:1231–1238
Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander–Tetri BA, Bhathal PS, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90
Koteish A, Yang S, Lin H, Huang X, Diehl AM. Chronic ethanol exposure potentiates lipopolysaccharide liver injury despite inhibiting Jun N-terminal kinase and caspase 3 activation. J Biol Chem. 2002;277:13037–13044
Nobili V, Carpino G, Alisi A, Franchitto A, Alpini G, De Vito R, et al. Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology. 2012;56:2142–2153
Yang S, Koteish A, Lin H, Huang J, Roskams T, Dawson V, et al. Oval cells compensate for damage and replicative senescence of mature hepatocytes in mice with fatty liver disease. Hepatology. 2004;39:403–411
Dorrell C, Erker L, Schug J, Kopp JL, Canaday PS, Fox AJ, et al. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev. 2011;25:1193–1203
Fausto N. Involvement of the innate immune system in liver regeneration and injury. J Hepatol. 2006;45:347
Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137(1478–1488):e1478
Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, et al. Epithelial–mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293–299
Zeisberg M, Hanai J-i, Sugimoto H, Mammoto T, Charytan D, Strutz F, et al. BMP-7 counteracts TGF-β1–induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968
Zeisberg M, Shah AA, Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem. 2005;280:8094–8100
Raiche J, Rodriguez-Juarez R, Pogribny I, Kovalchuk O. Sex-and tissue-specific expression of maintenance and de novo DNA methyltransferases upon low dose X-irradiation in mice. Biochem Biophys Res Commun. 2004;325:39–47.
Kulig E, Landefeld T, Lloyd R. The effects of estrogen on prolactin gene methylation in normal and neoplastic rat pituitary tissues. Am j pathol. 1992;140:207
Koturbash I, Kutanzi K, Hendrickson K, Rodriguez-Juarez R, Kogosov D, Kovalchuk O. Radiation-induced bystander effects in vivo are sex specific. Mutation Res/Fund Mol Mech Mutagenesis. 2008;642:28–36
Jung Y, Brown KD, Witek RP, Omenetti A, Yang L, Vandongen M, et al. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology. 2008;134:1532–1543
Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106
Choi SS, Omenetti A, Witek RP, Moylan CA, Syn W-K, Jung Y, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Ame J Physiol-Gastrointest Liver Physiol. 2009;297:G1093–G1106
Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, et al. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut. 2008;57:1275–1282
Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012-0007141) and the Research Fund Program of Research Institute for Basic Sciences, Pusan National University, Korea, 2009, project no. RIBS-PNU-2010-305.
Compliance with Ethical Requirements
Animal care and surgical procedures were approved by the Pusan National University Institutional Animal Care and Use Committee and carried out in accordance with the provisions of the NIH Guide for the Care and Use of Laboratory Animals.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Sihyung Wang and Keumju Lee contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, S., Lee, K., Hyun, J. et al. Hedgehog signaling influences gender-specific response of liver to radiation in mice. Hepatol Int 7, 1065–1074 (2013). https://doi.org/10.1007/s12072-013-9461-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-013-9461-0