Abstract
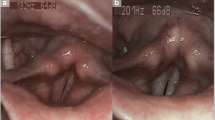
To evaluate treatment response in patients with laryngopharyngeal reflux (LPR). A prospective study of 100 patients with voice disorders was conducted. Patients were evaluated using reflux symptom index (RSI) and reflux finding score (RFS) by 70° rigid laryngoscope. Patients with RFS score of 7 or more were diagnosed of having LPR and were started with anti-reflux therapy for a period of 6 months. Patients were assessed at regular intervals using RSI and RFS. The prevalence of LPR in patients with voice disorders was found to be 25%. The mean RSI score improved gradually and significantly over a period of 6 months from 11.84 at presentation to 2.04 after 6 months of treatment (p value <0.001). The mean value of RFS improved from 7.92 at entry to 1.52 after 6 months of treatment (p value <0.001). However, it was found that the improvement was not significant at end of first month of treatment, and improvement in RSI and RFS scores was found only after 2 months of treatment. RSI and RFS improve significantly after treatment for 6 months with PPI like Omeprazole. But the improvement starts from the 2nd month from the onset of treatment. Treatment of LPR for at least 6 months may be indicated to attain a full resolution of physical findings.


Similar content being viewed by others
References
Vaezi MF (2003) Extraesophageal manifestations of gastroesophageal reflux disease. Clin Cornerstone 5:32–38
Park W, Hicks DM, Khandwala F et al (2005) Laryngopharyngeal reflux: prospective cohort study evaluating optimal dose of proton-pump inhibitor therapy and pretherapy predictors of response. Laryngoscope 115:1230–1238
Belafsky PC, Postma GN, Koufman JA (2001) The validity and reliability of the reflux finding score (RFS). Laryngoscope 111(8):1313–1317
Katz PO, Castell DO (2000) Medical therapy of supra- esophageal gastroesophageal reflux disease. Am J Med 108(suppl 4a):170S–177S
Steward DL, Wilson KM, Kelly DH et al (2004) Proton pump inhibitor therapy for chronic laryngopharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg 131:342–350
Habermann W, Schmid C, Neumann K, Devaney T, Hammer HF (2012) Reflux symptom index and reflux finding score in otolaryngologic practice. J Voice 26(3):e123–e127
Rouev P, Chakarski I, Doskov D, Dimov G, Staykova E (2005) Laryngopharyngeal symptoms and gastroesophageal reflux disease. J Voice 19(3):476–480
Belafsky PC, Postma GN, Koufman JA (2001) Laryngopharyngeal reflux symptoms improve before changes in physical findings. Laryngoscope 111(6):979–981
Koufman J, Sataloff RT, Toohill R (1996) Laryngopharyngeal reflux: consensus conference report. J Voice 10(3):215–216
Noordzij JP, Khidr A, Evans BA, Desper E, Mittal RK, Reibel JF et al (2001) Evaluation of omeprazole in the treatment of reflux laryngitis: a prospective, placebo-controlled, randomized, double-blind study. Laryngoscope 111(12):2147–2151
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All Authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Joshi, A.A., Chiplunkar, B.G. & Bradoo, R.A. Assessment of treatment response in patients with laryngopharyngeal reflux. Indian J Otolaryngol Head Neck Surg 69, 77–80 (2017). https://doi.org/10.1007/s12070-016-1046-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-016-1046-5