Abstract
Careful donor quality assessment and size match can impact long-term survival in lung transplantation. With this article, we review the conceptual and practical aspects of the preoperative donor lung quality assessment and size matching.
Similar content being viewed by others
Introduction
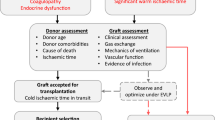
Once a donor is available, the blood type–matched recipients listed at different hospitals within 250 nautical miles of the donor hospital start receiving offers in the chronological order of allocation score. The allocation system is designed to prioritize organ allocation to sickest patients minimizing the ischemia time that occurred by travel; however, the system has been continuously debated, criticized, and modified [1,2,3]. In the USA, the hospital having the highest lung allocation score recipient within 250 nautical miles of donor location gets the offer first.
Upon receiving a call from Organ Procurement Organization (OPO) for a donor offer, the physician assesses the quality of the donor first and then determines the size matching. If the quality of the organ is perceived to be suboptimal or the primary recipient is found to be a size mismatch, the offer trickles down to other recipients listed at different hospitals within 250 nautical mile radius in the descending order of lung allocation score. After the 250 nautical mile list is exhausted, the offer is extended to a larger radius.
If donor lung quality and size match are acceptable, the offer is accepted provisionally by that institute after confirming an acceptable virtual cross-match. The donor risk profile is discussed with the recipient in detail and once the recipient agrees, the institute then sends a procurement team to the donor hospital and the final acceptance is decided after performing an intraoperative assessment.
With this article, we review the conceptual and practical aspects of the preoperative donor lung quality assessment and size matching.
Donor quality assessment
In order to assess the quality of the donor, the following things are taken into consideration—history of current admission, past medical history, past surgical history, cause of death, the most recent chest x-ray, and computed tomography (CT) scan, bronchoscopy, sputum cultures and most recent blood reports along with arterial blood gases according to standardized protocol mentioned in the “Donor management protocol” article of this special issue.
An ideal or standard donor criteria (SDC) guidelines (Table 1) were originally drafted in 1993 [4], and have remained largely unchanged since then [5]. Extended donor criteria (EDC) refers to the use of donor lungs that do not meet the standard criteria for transplantation; however, EDC does not imply marginal or sub-standard quality [6]. The definition of EDC is not uniform and varies among institutes [7,8,9].
We discuss practical situations pertaining to extended criteria donors and review the relevant literature.
Age
In the last 5 years, the International Society of Heart and Lung Transplantation (ISHLT) registry has shown an increased usage of donors older than 55 years. In Europe, the percentage of donors over 55 has steadily increased, with 34% of donors over the age of 55, and 21% older than 60 [14].
Multiple centers have reported their encouraging outcome suggesting that old non-smoker or less frequent smoking donor lungs may be acceptable [15,16,17]. Hecker et al. [16] reported that very old donor (> 70 years) with a lower percentage of smoking history and short ventilation time, had non-inferior survival compared to other donor age groups. Renard et al. [17] reported that carefully selected lung grafts from donors above 65 years of age are associated with similar outcomes compared to younger donors. Sommer et al. [15] reported that usage of donor lungs aged > 70 years is safe and showed better functional outcomes when used in emphysema patients.
In a larger United Network of Organ Sharing (UNOS) database analysis, Hall et al. [18] found that donors older than 60 years had the worst survival compared to all other donor age ranges in unadjusted analysis. However, the study also suggested that a significantly higher number of older donors were chosen for older recipients, which is an extremely common practice. Upon performing propensity-matched analysis based on relevant covariates for older recipients, they found no difference in survival [18].
Analyzing 18,673 patients in the UNOS registry, Chambers et al. [19] reported that the hazard ratio of 10-year mortality increases significantly with recipient’s age while donor’s age had a very minimal impact (Fig. 1). However, the risk of death while waiting for the lung transplant must be able to justify the use of a donor older than the recipient.
Hazard ratio for 10-year mortality for adult lung transplant recipients by recipient and donor age (transplants: 2000–June 2008, N = 18,673). This model excludes size match. The reference for recipient age is 54 years and for donor age is 36 years (reused with permission from Chambers et al. [19])
Cause of death
Drug overdose of intracranial bleed
Approximately 95% of lungs utilized for transplant in the USA come from brain-dead donors. The majority of brain-dead donors in the USA have died of drug overdose or intracranial bleed. Typically, after a drug overdose or sudden onset of intracranial bleed, the patient is found unconscious by a family member or bystander, and cardiopulmonary resuscitation is initiated by them or by emergency medical service. Because of profound hypoxia, brain sustains irreversible hypoxic injury resulting in brain death. The patients may have a history of profound vomiting secondary to a rise in intracranial tension caused by CO2 retention or intracranial bleed. Sometimes, emergency medical service responders may have to utilize the laryngeal airway at the time of cardiopulmonary resuscitation because of oral secretions or vomiting. These patients are at risk of developing aspiration pneumonia. A careful consideration of history and evaluation of CT scan can help to rule out aspiration pneumonia.
Trauma
Traumatic head injury leading to brain death comprises a significant portion of donors. Commonly, the donors have concomitant blunt chest wall trauma. Most common manifestation of blunt trauma is pulmonary contusion. Oftentimes, the patients require chest tube insertion for pneumothorax at the time of initial presentation; however, this should not preclude from a donation in many cases as majority of small lung injuries heal in 24–48 h of initial presentation and air leak can no longer be observed in the chest tube [20]. Persistent pneumothorax, subcutaneous emphysema, or air leak may adversely affect suitability. A careful intraoperative evaluation at the time of procurement is very important in this situation. In carefully selected cases, the outcomes are acceptable [20]. Massive blood transfusion and disseminated intravascular coagulation (DIC) is a frequent accompaniment of significant head injury, occurring up to 28% of all organ donors [21]. DIC may result in major organ dysfunction, most notably the lungs. Despite that, in absence of serious thrombosis or hemorrhage, organs may be accepted in selected cases [22].
Hanging
Lung allografts from hanging donors are historically considered marginal. It is believed that post-obstructive barotrauma and injury to alveolar-capillary barrier increases capillary permeability [23, 24]. Moreover, when the compression is suddenly removed, a prompt variation in venous return and intrathoracic pressure leads to reperfusion pulmonary edema with hyperemia [23, 24]. Bennett et al. suggested that hanging donors may have higher pulmonary vascular resistance and higher peak airway pressure (PAP) [25]. Most centers are very conservative when selecting these types of donors [24, 25]. However, contrary to common belief, allografts from hanging donors may be usable [25] and no statistically significant difference is reported at least in short-term and mid-term survival between hanging donor versus conventional donors [24, 26,27,28].
Drowning
Death by submersion or drowning is another debatable topic. As per UNOS database, a very few number of transplants happened from donors died of drowning [26] suggesting scarcity of data. Following a drowning event, voluntary apnea occurs which is only sustained temporarily. With eventual rise in alveolar CO2 levels, involuntary breathing happens (sometimes because of preceding neurological damage secondary to hypoxia), often leading to aspiration. On the other hand, approximately 10–15% of individuals develop severe and sustained laryngospasm, preventing aspiration of water or gastric contents—“dry drowning” [26]. Cooler temperature, dry drowning, microbiologically sparse environment (i.e., Alaska or Himalayas), deeper bodies of water, and lack of preceding neurological damage are favorable donor criteria following drowning [29]. Objectively, if the imaging does not show any evidence of aspiration or lung edema and pO2/FiO2 ratio is good enough, a bronchoscopy can help to determine the evidence of aspiration [26, 29].
Smoking
Tobacco smoking is widely prevalent amongst all age groups, hence amongst the majority of donors. Selection of donors with a smoking history of more than 20 pack-years is typically not advised [10]. However, due to the scarcity of available allografts, the risks of smoking have to be weighed against the risks of wait-list mortality [30]. The effects of smoking on lung function over the time after lung transplant are not very well described [30]. Conflicting evidence exists regarding usage of smoker donors, some studies showed similar survival [7, 31] while others showed worse survival [30] and some suggested impaired short-term survival but similar mid-term outcomes [32]. A combination of older donor and smoking history of about 20-pack-years may result in inferior outcomes [30]. Objectively, differences in parameters of small airway obstruction and pulmonary function test (PFT) at 8 weeks postoperatively are not significant between the donors with smoking history and those without smoking history [33].
Despite encouraging evidence, one should be carefully selecting the donors having more than 20 pack-years history of smoking. Clearly, donors with emphysematous lungs must be avoided. A careful evaluation of CT scan will help to rule out emphysematous changes (Fig. 2). Many donors with extensive smoking history do not develop emphysematous changes. In such cases, decision should be based on the urgency of the transplant and multiple other factors like donor age, gas exchange, length of intubation, bronchoscopy findings, etc. At the time of procurement, a careful evaluation of all areas, especially apices, must be done to rule out bullous or emphysematous changes.
Bronchial asthma
Theoretically, lung transplantation should denervate the donor lungs and bronchial asthma should not be a problem in the recipient. However, no scientific data is available on donor-derived bronchial asthma. This selection of lungs from the donors having a history of bronchial asthma remains subjective and largely depends upon the aggressiveness of the program.
Three distinct categories can be defined in the donors having a history of bronchial asthma.
-
1.
The donors who died of status asthmaticus—such donors typically have hypercarbia and moderate to severely elevated PAP. It is not recommended to use such lungs for transplantation purposes [34].
-
2.
Well-controlled bronchial asthma on frequent or regular medical treatment for bronchial asthma—such donors are classified as high risk [35], therefore, usually not preferred. The risk of utilizing such donor lungs must be justified by severity of the recipient’s disease.
-
3.
The donors who had a history of bronchial asthma, not on treatment or requiring infrequent treatment, or having history of exercise-induced asthma—such donors are usually classified as moderate risk [35] but can be accepted in selected cases with reasonable outcomes [34].
Marginal oxygenation
Prolonged ventilation (> 7 days) and tracheostomy are red flags but not absolute contraindications. With prolonged ventilation in a supine position, donors frequently develop basal atelectasis and mucus plugs resulting in suboptimal gas exchange [36]. In this scenario, one should seek for the serial values of paO2/FiO2 ratio during the entire admission. If paO2/FiO2 ratio in any of the blood gas is more than 400 and the most recent CT scan only suggests atelectasis, the lungs may be usable. Moreover, bronchoscopy findings also help to differentiate between atelectasis versus pneumonia. Mucus plugs can be removed using bronchoscopy and atelectasis can be treated by Valsalva. Frankly purulent secretions with immediate repooling from the distal airways would suggest more towards pneumonia.
Because of dependent atelectasis, intraoperative paO2/FiO2 ratio is usually higher than the one recorded in the intensive care unit (ICU) from the arterial line [36]. A hard cut-off of paO2/FiO2 ratio at 300 could result in up to 36% of loss in donor organs [36].
Pneumonia or severe atelectasis results in intrapulmonary shunting of the blood giving suboptimal paO2/FiO2 ratio in systemic arterial blood gas. In such cases, individual pulmonary vein blood gases can help to determine the quality of the contralateral lung.
In morbidly obese patients, bilateral dense basal atelectasis, secondary to chest wall strapping phenomena [37], often results in suboptimal arterial blood gases. Majority of times, after opening the donor’s chest and providing Valsalva, the atelectasis goes away and paO2/FiO2 ratio improves [38].
Ventilator parameters and lung compliance assessment
In order to achieve uniformity, OPOs have standardized ventilator management on lung donors in USA. At the time of offer, physicians must verify the donor’s tidal volume, positive end-expiratory pressure, respiratory rate, and ventilator FiO2. In addition to the assessment of paO2/FiO2 ratio, analyzing the lung compliance can provide an excellent idea about any existing pulmonary disease or pulmonary edema. Generally, prolonged ventilation and tracheostomy are not prohibitive for lung donation provided the quality is perceived as adequate otherwise.
Compliance is defined as the change in volume that occurs per unit change in the pressure of the system. For practical purposes, the donor lung compliance is assessed by PAP and plateau pressure (Pplat). PAP above 25 cm of water and Pplat above 20 cm water may suggest lung edema or airway obstruction.
Infection
Growth of multidrug-resistant Staphylococcus aureus or other multi-drug resistant bacteria with radiographic evidence of extensive pneumonia bars the usage of the donor lungs. However, in absence of significant radiographic evidence of pneumonia, positive bronchial cultures for drug sensitive bacteria should not have any significant impact on 30 day mortality [39, 40], primary graft dysfunction [40], or ICU stay [39]. Recently, a single center study from Korea reported that high levels of preexisting multi-drug resistant bacteria in donor lung allografts does not increase chances of early peritransplant pneumonia and mortality rates [41]; however, this practice is not widely adopted.
Positive blood culture should not be considered as an absolute contraindication to transplantation. The recipient should receive appropriate antibiotics during the peritransplant period to minimize the potential risk of transmission of infection [42].
Malignancy
Any history of malignancy should raise an alarm. An active malignancy in the donor usually precludes the organ donation as the Cincinnati Transplant Tumor Registry reported an unacceptably high rate of tumor transmission in the patients receiving organs from the donors with malignancies [43]. Only localized and excised skin (commonly basal cell carcinoma) or in situ cervical cancer, and primary central nervous system malignancies that rarely metastasize, albeit in the absence of major surgical excision or placement of intraventricular shunts, may be suitable for organ donation [10]. A duration of remission and oncology expert opinion could provide reasonable confidence in decision making. In such cases, details of chemotherapy and radiation must be extracted as drugs like bleomycin and chest radiation can cause pulmonary fibrosis.
Hepatitis C virus-infected donors.
Transplantation of organs from hepatitis C virus (HCV)–infected donors into uninfected recipients typically leads to chronic HCV infection in up to 82% of recipients [44]. With development of potent direct-acting antiviral agents to treat HCV infection has provided an opportunity to treat such infection in the recipients. However, this practice is not widely accepted yet for lung transplant.
A nucleic acid test (NAT) is performed in donors with a positive antibody test to rule out viremia. Although a negative NAT rules out viremia and implies that the risk of infection transmission from such donors is negligible [45], centers typically have been conservative about selecting donors with positive antibody test because of lack of data on long-term outcomes.
Some centers have been very aggressive even for NAT-positive donors. Recently, in a single center (DONATE HCV) trial, Woolley et al. [44] reported successful treatment of all donor-derived HCV infection and acceptable short-term outcomes [46]. Typically, after the transplant from NAT positive donors, antiviral treatment is started within few hours once the recipient becomes stable enough.
Donation after cardiac death
Although donation after cardiac death (DCD) donors have been slowly increasing, the majority of centers are still conservative and DCD transplant remains a minority of transplants performed in the USA [47]. Carefully selected DCD lungs provide excellent outcomes [47]. Details about DCD donors are not included in this article.
Donor lung score
Multiple donor lung score calculators were developed in order to objectively quantify the donor lung quality [35, 48,49,50]. However, a large-scale validation and hence utilization of such scores are not done yet. The existing literature mentions retrospective score calculation and outcome analysis based on that. Prospective trials have not been done yet comparing the donor lung score versus intuitive manual evaluation.
Notably, due to population heterogenicity, utilization of European lung donor score may not be useful in the USA or Asian countries or vice versa. Majority of such scores do not include the most important parameters which require subjective assessment (i.e., CT scan, X-ray, or bronchoscopy evaluation). Moreover, they may not be very practical, especially for high-volume centers, which generally receive approximately 1000–3000 lung offers every year.
Screening for active Corona Virus Disease 2019 infection
In the era of the Corona Virus Disease 2019 (COVID-19) pandemic, institutional protocol to rule out COVID-19 infected donors must be established and modified as needed as the knowledge base is dynamically expanding [51]. Recently, the organ procurement and transplant network published a summary of current evidence and information regarding donor COVID-19 testing in organ recovery [52]. According to the report, an active COVID-19 infection is defined as “an immunocompetent donor with a history of confirmed COVID-19, 21 days from the date of disease onset and COVID-19 detected in a respiratory sample or an asymptomatic donor with detection of COVID-19 in a respiratory sample without reliable history” [52]. The ISHLT also published a revision of guidelines on February 1 [53].
UNOS and OPOs in the USA have provided guidelines for preoperative screening of COVID-19 symptoms in donors [53].
At our institute, we consider following protocol. The donors must have at least 2 separate COVID-19 tests, one of them must be bronchoalveolar lavage specimen, and a non-contrast high-resolution CT scan within 48 h of the offer. Even if the donor’s COVID-19 test is negative, information like donor’s recent direct or indirect exposure to COVID-19 positive person, number of COVID-19 patients in donor’s ICU, the overall prevalence of COVID-19 in the region, and isolation protocols of the hospital are reviewed carefully. It is not uncommon to find ground-glass opacities (GGO) and tree-in-bud appearance in CT scan without a positive COVID test. COVID-19 infection risk for the donor must be stratified in such cases [54]. One should be extremely careful in selecting donors with GGO given the unclear picture of sensitivity and specificity of COVID-19 test.
Resolved COVID-19 infection
With extensive community spread of COVID-19 and early development of herd immunity, the number of donors having a history of COVID-19 infection is rising. Majority of them fully recovered from COVID-19 infection and CT scan usually does not show any evidence of ground-glass opacity after few weeks. Resolved COVID-19 donor is defined as “an immunocompetent donor with a history of confirmed COVID-19 with a resolution of symptoms and more than 21 days from the date of onset of symptoms” [52]. The donors with resolved COVID-19 and a negative NAT at the time of donor evaluation are unlikely to transmit the infection. The donors with resolved COVID-19 and a positive NAT from 21 to 90 days after the date of disease onset are unlikely to transmit the infection [52, 53]. A positive NAT likely represents a nonviable virus in such cases. However, a positive NAT after 90 days of the disease onset may reflect a reinfection. Moreover, long-term implications of lung parenchymal changes after COVID-19 infection are not clear and at this moment, we usually defer using the donors having previous COVID-19 infection.
The decision to recover and transplant organs in such cases should include two things: (1) Recipient risk of mortality or further complications while delaying the transplantation and remaining on waiting list. (2) Current unknown long-term outcomes from the donor with history of resolved COVID-19 [52].
Practical considerations for donor quality assessment
The success in lung transplant is lower compared to that of other solid organs in terms of post-transplant outcomes and probably because of that, programs have been relatively conservative in accepting marginal donors. So, the programs frequently face the dilemma between accepting marginal donors and wait-list mortality. Over the past decade, there has been an increase in absolute number of lung transplantations performed in the USA, with a slight decrease in waitlist mortality to 17.2/100 waitlist years [55]. Wait-list mortality is the highest amongst patients with pulmonary fibrosis, cystic fibrosis, and pulmonary arterial hypertension, and is lowest in the patients with emphysema [56]. Relaxing donor selection criteria can help centers to cope with the shortage of supply of donor lungs.
According to the data from organ procurement and transplantation network/scientific registry for transplant recipients, an increase in the frequency of DCD lung donation is noted recently [55]. Unfortunately, the number of discarded lungs has also increased [55] and the donor utilization rate remains near 19% [57]. In the last two decades, the transplant programs underwent continuous evolution in donor organ acceptance practices using EDC, significantly improving donor utilization rates, in some centers up to 70% [12, 13]. From the UNOS registry data, 56% of donor lungs used varied from the SDC criteria by at least one count, 41% had chest X-ray abnormalities, 18% had PaO2 less 300 mmHg, and 21% had a history of smoking more than 20 packs years [5].
Waitlist mortality is highest in the pulmonary fibrosis group as the disease progresses very rapidly after a certain time and results in a rapid decline in clinical course [56]. With a rapid decline in respiratory status, pulmonary fibrosis patients often demonstrate lesser preoperative physical endurance making them sicker patient population compared to chronic obstructive pulmonary disease (COPD) patients. High volume centers frequently relax their donor selection criteria for sick pulmonary fibrosis patients, especially when organ offer is coming from a nearby hospital, minimizing travel time and travel expenses in case of failure to utilize those lungs upon onsite evaluation.
Results of EDC lungs
Using different EDC definitions and various ex vivo perfusion systems, groups have reported acceptable [7, 8, 12, 13, 58,59,60] outcomes.
EDC lungs have been associated with higher primary graft dysfunction [7] and an increase in ICU stay [58] but have shown no impact on acute rejection [7, 58, 59], chronic rejection [59], and long-term survival [58, 59]. Analyzing 3792 EDC donations from the UNOS database, Mulligan et al. [8] determined that EDC is associated with lower 1-year survival in patients having lung allocation score > 70 compared to SDC.
Although the number of hospitals adopting extended criteria donors is increasing, further data regarding risks and survival benefits are awaited. At this moment, severity, acuteness, and short-term prognosis of the recipient’s disease must be able to justify the usage of extended criteria donors.
Recently, several donor score systems have been suggested for quality assessment [13, 14]. The scoring system may be useful in retrospective analysis/quantification of donor quality. However, practical use of such system may not be widely adopted given the complexity and lack of uniformity.
Ethics
Key factors are often ignored while debating the EDC—patient autonomy, education, and consent for the usage of EDC lungs. High priority is always given to the patients having the highest lung allocation score, who are likely to die without transplant and less likely to survive the operation. These patients are likely to provide consent for EDC use [6]. However, low priority candidates (lung allocation score < 50), comprising of a majority of transplant recipients, demonstrates lesser survival benefit compared to mid-priority candidates (Lung Allocation Score 50–79) given the low risk of death on waiting list [61]. Therefore, risks of the EDC must be discussed with patients having low allocation score.
Size matching
In the early years of lung transplantation, pioneering surgeons were more concerned with surgical anastomoses and infection than the potential size disparity of lung volume between recipient and donor [62]. Therefore, in the 1980s, little or no attention was given to the idea of size matching donor and recipient lungs [63]. In 1992, the American Thoracic Society workshop on lung transplantation recommended using allografts with “similar total lung capacities” between donor and recipient [64, 65].
Here, we explain the conceptual understanding of size matching and describe practical considerations.
Three aspects of size-matching
-
1.
Surgical feasibility
From the surgeon’s point of view, appropriately size-matched lungs should not have major discrepancies in the bronchus, pulmonary artery, and pulmonary vein sizes. A large discrepancy will make the bronchial anastomosis not only difficult but will also increase the chances of stenosis or dehiscence. Too small lungs in a large chest cavity lead to an elevated risk of pneumothorax, hemothorax, and bleeding after surgery while large lungs in a small chest cavity will lead to increased incidence of the open chest and surgical wound complications [66].
-
2.
Respiratory mechanics
Pleural cavity has a negative pressure that helps with lung expansion and compliance. Because of this negative pressure, chest wall conforms around the lung parenchyma in chronic diseases like COPD, and idiopathic pulmonary fibrosis (IPF). In patients with IPF, the chest cavity becomes small secondary to shrinking lung parenchyma. The diaphragm moves up and rib spaces are crowded as the total lung capacity (TLC) reduces with the time when the disease progresses. On the other hand, in patients with COPD, chest cavity is large, diaphragm remains flat, and the rib spaces are widened as the disease progresses and TLC increases. Both diseases create inefficient respiratory mechanics, contributing to shortness of breath (Fig. 3). Ideally, an appropriate size-matched lung should be able to restore the respiratory mechanics back to normal.
-
3.
Circulatory physiology
Physiologically, the lungs cannot be seen separately from the cardiovascular system as both pulmonary overflow and underflow situations could affect long-term lung function. Cardiac output parallels to body surface area which depends on both height and weight. In an ideal world, the donor and recipient body surface area should match very closely in order to achieve a good size-match from a circulatory standpoint. The pulmonary vasculature is usually very compliant and can tolerate increased flow without any immediate detrimental effects as seen in atrial septal defect physiology. However, in cases of severe pulmonary arterial hypertension undergoing bilateral lung transplants, cardiac output increases significantly immediately after the transplant. A sudden increase in flow through the pulmonary circulation is considered as one of the major risk factors for higher incidence of primary graft dysfunction in this diagnosis group [67]. Eberlein et al. [68] speculate that a larger pulmonary vascular bed and larger lung volume would minimize global and regional pulmonary over-distention. These changes would thereby minimize right ventricular after-load by providing the optimally lowest pulmonary vascular resistance and may result in less endothelial injury and dysfunction, improving overall survival [69].
How to estimate lung size?
The big question remains. How do we measure the chest wall size objectively? In normal individuals, TLC is equal to chest wall cavity size. In patients with IPF or COPD, actual TLC (aTLC), as measured by PFT, is significantly different from the predicted or expected measurements and does not reflect the true physiological lung parenchyma volume sought after transplant. Moreover, for donors, PFT data is not available. So, population-based-predicted TLC (pTLC) formula could help here.
pTLC formula
pTLC is calculated on the basis of a person’s gender and height. It predicts the TLC and hence the chest wall cavity size on the basis of population data. The most commonly used formula was described by Stocks and colleagues in 1995 [70]:
pTLC for men = [7.99 × height in meters] – 7.08.
pTLC for women = [6.60 × height in meters] – 5.79.
A ratio of aTLC and pTLC provides a quantitative estimate of chest cavity distortion secondary to the disease in the recipient. In severe COPD patients, aTLC could be as high as 150–175% of predicted. Similarly, in severe fibrosis patients, aTLC could be as low as 50% of predicted.
Size-match definition.
Currently, the most widely accepted size match definition uses the pTLC formula as mentioned above [66, 68, 71,72,73]. The ratio between donor and recipient pTLC is calculated. If the ratio is < 1 the donor is considered undersized and if it is > 1, the donor is considered oversized. Despite significant limitations, this formula is considered a gold standard to define the size match at present.
The ISHLT consensus statement in 2003 suggested an allograft for double lung transplant should be between 75 and 125% of the recipient’s pTLC, with the caveat that larger organs are preferred for patients with pre-operative hyperinflation and smaller organs for patients with a pre-operative restrictive impairment [10].
However, there are several limitations of this popular pTLC calculation formula.
-
1.
It is based on small number of white male and white female patients [70, 71]. It does not include the racial differences between lung size. For example, African American individuals may have up to 12% smaller chest cavity size [70, 74]. However, the data is anecdotal and inter-racial comparison of pTLC formula is not available.
-
2.
It does not include correction of mild to moderate overinflation that smoker donors often have.
-
3.
It does not include the weight of a person. It is frequently observed that lungs from a severely obese donor are larger than expected after opening the chest because of chest wall strapping phenomena [37].
Practical considerations
Although, size matching is defined as pTLC ratio, majority of centers worldwide do not consider the pTLC ratio practical because of multiracial pool of the donors/recipients, limitations of pTLC formula and cumbersome calculation. Height difference, chest wall measurements, and intuition are the most common way of assessing the size match.
Height difference
As mentioned above, height remains the most important factor for pTLC calculation. Therefore, height matching should be roughly parallel to the pTLC ratio in gender-matched situations. In cases of gender mismatch, it is broadly considered that female lungs are 20% less in volume than height-matched male. These assumptions make the size match practically easier.
Chest wall measurements
In recipients having severe fibrosis, the chest wall cavity is shrunken extremely. Putting an oversized lung in a small chest cavity may require the surgeon to leave the chest open. It may not only increase the postoperative wound complications but also increase the ventilation time and overall postoperative stay. In some cases, significantly oversized lungs may require lobectomy also. Therefore, the majority of centers in the USA also consider chest wall measurements while size-matching. For this reason, the donor’s chest x-ray measurements are routinely provided by OPOs.
Most frequently, the measurements are obtained using a digital chest x-ray. Both vertical and horizontal dimensions are considered commonly (Fig. 4). It is worth noting here that the recipient's chest x-ray is in a standing position without positive pressure ventilation and donor’s chest X-ray is in supine position with positive pressure ventilation. Also, as mentioned above, recipient’s chest cavity is frequently significantly distorted given the underlying disease. So, a combination of chest wall measurements along with the assessment of the height discrepancy remains the cornerstone for size-matching at our institute.
All recipients must have lateral chest X-ray. Recipient’s antero-posterior diameter of the chest wall cavity is also important. Many recipients, despite being tall, have shallow chest cavities. Deeper (larger antero-posterior diameter) chest cavities tend to easily accommodate larger lungs.
Body mass index
Severe obesity is associated with chest wall strapping phenomena [37]. It is frequently observed that after opening pleura of obese donors, lungs are found bigger than they are perceived in CT scan. The impact of chest wall strapping phenomena on pTLC calculation is not measured in any of the studies [37].
Age
In teenage donors, it is frequently perceived intraoperatively that lung size is significantly smaller than other height-matched adults. There is no pTLC formula for teenage donors. Chest wall dimensions and CT scan examination remains the mainstay for size-matching. Fortunately, teenage lungs are usually very compliant, and they can adapt to a wide range of chest cavity sizes.
Size matching in bilateral lung transplant
Primary pulmonary hypertension
Unlike COPD or fibrosis, primary pulmonary arterial hypertension may not affect the chest wall size. Eberlein et al. [68] reported that oversized donors (pTLC ratio > 1) are associated with better survival.
Fibrosis
For IPF patients with shrunken chest cavities and moderate to severely low TLC, usually, 1–3 in. shorter donors are preferred at our hospital. Although, it would not be the ideal size match considering pTLC based size-match definition, but in the last decade, majority of centers have shifted from pTLC based formula to more practical way of size matching using height and chest x-ray measurements-based matching. Significantly oversized bilateral lung implantation could increase the chances of difficult chest closure, especially with clamshell incision.
COPD
In COPD patients, chest cavity size is significantly larger than in normal persons. Implanting a significantly larger lung allograft may not result in normalization of respiratory mechanics and may impact negatively on physical endurance or 6-min walk distance post-transplant and survival [75].
Mason et al. reported that the usage of extremely small (donor’s pTLC less than 67% of recipient’s aTLC) or extremely large (donor’s pTLC more than recipient’s aTLC) donor lungs in emphysema patients results in inferior survival with bilateral lung transplantation [75]. This finding indirectly supports the hypothesis of the importance of normalization of respiratory mechanics after bilateral lung transplantation (Fig. 5).
A Preoperative chest x-ray for 67 in. tall male COPD patient. B Postoperative chest X-ray of the same patient after receiving bilateral lung transplant from 67 in. tall male donor. In this example, restoration of chest cavity size and mechanics is very well observed. C Preoperative chest x-ray for 62 in. tall female COPD patient. D Postoperative chest X-ray of the same patient after receiving bilateral lung transplant from 69 in a tall female donor. In this example, persistent hyperinflation changes of the chest wall can be seen even after the transplantation suggesting significant oversizing of donor lungs
Size matching in single lung transplant
Primary pulmonary hypertension
Majority of centers choose to perform double lung transplantation with severe pulmonary arterial hypertension. However, in patients with mild-to-moderate pulmonary arterial hypertension and in selected cases of severe secondary pulmonary arterial hypertension, single lung transplantation provides similar survival benefit [76].
In patients with pulmonary hypertension, evaluation of preoperative perfusion scan is very important. Usually, the side with the worst perfusion is chosen for the transplant. In such cases, the transplanted lung remains at risk of congestion and pulmonary over-circulation and hence primary graft dysfunction as it will have lower pulmonary vascular resistance than the native contralateral lung. Therefore, slightly oversized lungs according to gender-matched height matching or pTLC formula are preferable as the larger lung, theoretically, will have better vascular compliance to accommodate for the increased pulmonary flow.
COPD
After a single lung transplant (SLT) in COPD patient, there is a substantial risk of allograft compression by over-inflation of the native lung, particularly with mechanical ventilation immediately after transplant (Fig. 6). Theoretically, in such cases, larger the lung allograft size, the lesser the compression would be. With extreme over-inflated chests, it is not uncommon to have up to 10 in. of difference in height between donor and recipient.
Recently, analyzing UNOS data from 2005 to 2017, the Columbia University group reported that single left lung transplant in COPD cases has inferior long-term survival compared to single right lung or double lung transplant [77]. However, in the same conference, Timofte et al. presented that there is no difference between survival of single or double lung transplant in COPD patients [78].
Previously, Brunsting et al. [79] had found no correlation between post-operative pulmonary function and estimated volume of donated lung tissue or relative donor-to-recipient size matching. Hayden et al. [80] also reported no correlation between donor and recipient size match (actual and predicted) and the degree of functional improvement after SLT. In addition, no significant differences were identified when comparing the functional outcomes of right and left SLT recipients [72].
Theoretically, as the COPD progresses in the native lung, it continues to compress the contralateral transplanted lung resulting in restrictive allograft dysfunction. However, Estenne et al. determined that compared with the ipsilateral lung in normal control subjects, although the TLC of the graft is substantially reduced, the functional residual capacity is within normal limits [81]. They also reported that the TLCs of the graft and of the native lung do not change over time after SLT for emphysema. Weill et al. [82] investigated acute native lung hyperinflation and found no evidence of an impact on FEV1 or 6-min walk results at 1 year or survival rates of acute rejection, infection, or BOS. They concluded that, although acute native lung hyperinflation is common radiographically, it is rarely clinically severe [82].
As a large volume center for lung transplantation, our experience parallels that of Columbia University group [77] suggesting outcomes of right lung transplantation are better than the left lung transplantation in COPD patients.
Fibrosis
Pulmonary fibrosis could be more severe on one side than the other. In such cases, the more severe side usually has smaller chest cavity. In such cases, complete reliance on height or predicted TLC-based formula could be catastrophic as the size of the recipient’s chest cavity is significantly less than the estimation (Fig. 4).
Since the native lung on the severe side is more dysfunctional, it would not be unreasonable to choose that side for transplantation. However, Tsagkaropoulos et al. reported that the transplant side does not influence recipient survival, freedom from bronchiolitis obliterans, complications, or pulmonary function after SLT [83]. Besides surgical considerations in the recipient, the offer of a donor lung opposite to the preferred side should not be a reason to postpone the transplantation until a better-matched donor is found [83].
Importance of size-matching and survival
Size-matching and waitlist mortality
Pulmonary fibrosis patients can have a rapid decline in clinical course compared to COPD patients, who remain typically stable over years. Moreover, pulmonary fibrosis patients have smaller chest cavity size. Therefore, shorter pulmonary fibrosis patients usually experience longer wait-times as the organ supply is slightly skewed towards taller donors [84]. Not a surprise, as a result of that, height remains the independent predictor factor for waitlist mortality [85]. Inclusion of height along with diagnosis in lung allocation score is being debated [85].
Size-matching and survival
In 2019, the focus theme for the 36th adult lung and heart–lung transplantation report was “Donor and recipient size match.” This report summarized data from 69,200 adult lung and 4,128 adult heart–lung transplants performed through June 30, 2018, and reported to the International Thoracic Organ Transplant Registry. The analysis showed that slightly taller and heavier donors have favorable survival outcome in lung transplantation [19] (Fig. 7). Analyzing the hazard ratio curves, it is evident that oversized donors in terms of height difference provide survival advantage, the advantage becomes flat when oversizing reaches up to the extreme end. However, weight difference does not have a very significant impact on survival. These findings also correlate with single-center studies [67, 71, 86].
A Large UNOS Database analysis. Top: Hazard ratio of 5-year mortality for adult lung transplant recipients by donor-recipient weight difference (transplants: 2005–June 2013, N = 27,023). The reference value for weight difference is 5 kg; Bottom: Hazard ratio of 5-year mortality for adult lung transplant recipients by donor-recipient height difference (transplants: 2005–June 2013, N = 27,203). The reference value for height difference is 2.4 cm (reused with permission from Chambers et al. [19])
Conclusion
Usage of extended criteria donors has increased significantly in the last few years with encouraging results. Considering multiple factors from both recipient’s and donor sides, size-matching remains more intuitive than objective. The current evidence suggests that with full consideration of surgical feasibility, implanting the largest possible lungs results in the best possible survival.
References
Al-Ebbini L, Oztekin A, Chen Y. FLAS: Fuzzy lung allocation system for US-based transplantations. Eur J Oper Res. 2016;248:1051–65. https://doi.org/10.1016/j.ejor.2015.08.001.
Auraen H, Schultz HHL, Hammainen P, et al. Urgent lung allocation system in the Scandiatransplant countries. J Heart Lung Transplant. 2018;37:1403–9. https://doi.org/10.1016/j.healun.2018.08.002.
Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212–27. https://doi.org/10.1111/j.1600-6143.2006.01276.x.
Sundaresan S, Trachiotis GD, Aoe M, Patterson GA, Cooper JD. Donor lung procurement: assessment and operative technique. Ann Thorac Surg. 1993;56:1409–13. https://doi.org/10.1016/0003-4975(93)90699-i.
Reyes KG, Mason DP, Thuita L, et al. Guidelines for donor lung selection: time for revision? Ann Thorac Surg. 2010;89:1756–64.
Neizer H, Singh GB, Gupta S, Singh SK. Addressing donor-organ shortages using extended criteria in lung transplantation. Ann Cardiothorac Surg. 2020;9:49–50. https://doi.org/10.21037/acs.2019.10.01.
Somers J, Ruttens D, Verleden SE, et al. A decade of extended-criteria lung donors in a single center: was it justified? Transpl Int. 2015;28:170–9. https://doi.org/10.1111/tri.12470.
Mulligan MJ, Sanchez PG, Evans CF, et al. The use of extended criteria donors decreases one-year survival in high-risk lung recipients: a review of the United Network of Organ Sharing Database. J Thorac Cardiovasc Surg. 2016;152:891–898.e2. https://doi.org/10.1016/j.jtcvs.2016.03.096.
Elmer A, Birrer M, Weiss J,et al. Extended-criteria donors in lung transplantation in Switzerland: an evaluation of two adapted lung donor scores. Swiss Med Wkly. 2018;148:w14614.
Orens JB, Boehler A, de Perrot M, et al. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. 2003;22:1183–200. https://doi.org/10.1016/s1053-2498(03)00096-2.
Raemdonck DV, Neyrinck A, Verleden GM, et al. Lung donor selection and management. Proc Am Thorac Soc. 2009;6:28–38. https://doi.org/10.1513/pats.200808-098GO.
Smail H, Saxena P, Wallinder A, et al. Donor lung procurement by surgical fellow with an expectation of high rate of lung utilisation. Heart Lung Circ. 2018;27:961–6.
Kotecha S, Hobson J, Fuller J, et al. Continued successful evolution of extended criteria donor lungs for transplantation. Ann Thorac Surg. 2017;104:1702–9. https://doi.org/10.1016/j.athoracsur.2017.05.042.
Smits JM, Gottlieb J, Verschuuren E, et al. Impact of donor lung quality on post-transplant recipient outcome in the Lung Allocation Score era in Eurotransplant–a historical prospective study. Transpl Int. 2020;33:544–54.
Sommer W, Ius F, Salman J, et al. Survival and spirometry outcomes after lung transplantation from donors aged 70 years and older. J Heart Lung Transplant. 2015;34:1325–33. https://doi.org/10.1016/j.healun.2015.06.002.
Hecker M, Hecker A, Kramm T, et al. Use of very old donors for lung transplantation: a dual-centre retrospective analysis. Eur J Cardiothorac Surg. 2017;52:1049–54. https://doi.org/10.1093/ejcts/ezx202.
Renard R, Girault A, Avramenko-Bouvier A, et al. Outcome of lung transplantation using grafts from donors over 65 years of age. Ann Thorac Surg. 2020. https://doi.org/10.1016/j.athoracsur.2020.10.018.
Hall DJ, Jeng EI, Gregg JA, et al. The impact of donor and recipient age: older lung transplant recipients do not require younger lungs. Ann Thorac Surg. 2019;107:868–76. https://doi.org/10.1016/j.athoracsur.2018.09.066.
Chambers DC, Cherikh WS, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019;38:1042–55. https://doi.org/10.1016/j.healun.2019.08.001.
Schwarz S, Rahimi N, Kifjak D, et al. Lungs from polytrauma donors with significant chest trauma can be safely used for transplantation. J Thorac Cardiovasc Surg. 2020. https://doi.org/10.1016/j.jtcvs.2020.10.150.
Nygaard CE, Townsend RN, Diamond DL. Organ donor management and organ outcome: a 6-year review from a Level I trauma center. J Trauma. 1990;30:728–32.
Frost AE. Donor criteria and evaluation. Clin Chest Med. 1997;18:231–7. https://doi.org/10.1016/s0272-5231(05)70374-9.
Weissman C, Damask MC, Yang J. Noncardiogenic pulmonary edema following laryngeal obstruction. Anesthesiology. 1984;60:163–5.
De Wolf J, Renard R, Lehouerou T, et al. Hanging donor lungs give good short-, mid- and long-term results in lung transplantation. Clin Transplant. 2020;34: e13758. https://doi.org/10.1111/ctr.13758.
Bennett DT, Reece TB, Smith PD, et al. Ex vivo lung perfusion allows successful transplantation of donor lungs from hanging victims. Ann Thorac Surg. 2014;98:1051–6.
Whitson BA, Hertz MI, Kelly RF, et al. Use of the donor lung after asphyxiation or drowning: effect on lung transplant recipients. Ann Thorac Surg. 2014;98:1145–51.
Mohite P, Patil N, Sabashnikov A et al., editors. “Hanging donors”: are we still skeptical about the lungs? Transplantation proceedings; 2015: Elsevier.
Ananiadou O, Schmack B, Zych B, et al. Suicidal hanging donors for lung transplantation: Is this chapter still closed? Midterm experience from a single center in United Kingdom. Medicine(Baltimore). 2018;97:e0064.
Pasupneti S, Patel K, Mooney JJ, Chhatwani L, Dhillon G, Weill D. Lung transplantation following death by drowning: a review of the current literature. Clin Transplant. 2016;30:1195–7. https://doi.org/10.1111/ctr.12822.
Schultz H, Møller C, Zemtsovski M et al., editors. Donor smoking and older age increases morbidity and mortality after lung transplantation. Transplantation Proceedings; 2017: Elsevier.
Sabashnikov A, Patil NP, Mohite PN, et al. Influence of donor smoking on midterm outcomes after lung transplantation. Ann Thorac Surg. 2014;97:1015–21. https://doi.org/10.1016/j.athoracsur.2013.11.020.
Okahara S, Levvey B, McDonald M, et al. The impact of donor tobacco and marijuana smoking history on early and intermediate-term lung transplant outcomes. J Heart Lung Transplant. 2020;39:S377-S.
Belousova N, Ma J, Wu J, et al. Effects of donor smoking history on early post-transplant lung function measured by oscillometry. J Heart Lung Transplant. 2020;39:S309–10.
Oto T, Griffiths A, Levvey B, et al. Donor history of asthma is not a contraindication to lung transplantation: 12-year single-center experience. J Heart Lung Transplant. 2004;23:309–16. https://doi.org/10.1016/s1053-2498(03)00192-x.
Loor G, Radosevich DM, Kelly RF, et al. The University of Minnesota Donor Lung Quality Index: a consensus-based scoring application improves donor lung use. Ann Thorac Surg. 2016;102:1156–65. https://doi.org/10.1016/j.athoracsur.2016.04.044.
Whitford H, Kure CE, Henriksen A, et al. A donor PaO2/FiO2 < 300 mm Hg does not determine graft function or survival after lung transplantation. J Heart Lung Transplant. 2020;39:53–61. https://doi.org/10.1016/j.healun.2019.08.021.
Eberlein M, Schmidt GA, Brower RG. Chest wall strapping. An old physiology experiment with new relevance to small airways diseases. Ann Am Thorac Soc. 2014;11:1258–66. https://doi.org/10.1513/AnnalsATS.201312-465OI.
Okamoto T, Omara M, Ahmad U, et al. Utilization of marginal lung donors with low PaO2/FiO2 ratio and high body mass index. Ann Thorac Surg. 2020;109:1663–9. https://doi.org/10.1016/j.athoracsur.2019.12.072.
Howell CK, Paciullo CA, Lyon GM, et al. Effect of positive perioperative donor and recipient respiratory bacterial cultures on early post‐transplant outcomes in lung transplant recipients. Transpl Infect Dis. 2017;19:e12760.
Ahmad O, Shafii AE, Mannino DM, Choate R, Baz MA. Impact of donor lung pathogenic bacteria on patient outcomes in the immediate post‐transplant period. Transpl Infect Dis. 2018;20:e12986.
Lee KH, Jeong SJ, Kim SY, et al. Effects of multidrug-resistant bacteria in donor lower respiratory tract on early posttransplant pneumonia in lung transplant recipients without pretransplant infection. Transplantation. 2020;104:e98–e106.
Shah SZ, Pouch SM, Keller BC, Pope-Harman A, Tumin D. Outcomes from bacteremic donors in lung transplantation. J Heart Lung Transplant. 2017;37:302–4. https://doi.org/10.1016/j.healun.2017.07.023.
Snell GI, Westall GP. Selection and management of the lung donor. Clin Chest Med. 2011;32:223–32. https://doi.org/10.1016/j.ccm.2011.02.002.
Woolley AE, Singh SK, Goldberg HJ, et al. Heart and lung transplants from HCV-infected donors to uninfected recipients. N Engl J Med. 2019;380:1606–17. https://doi.org/10.1056/NEJMoa1812406.
Watson J, Mulvihill MS, Cox ML, et al. Early experience with the use of hepatitis C antibody-positive, nucleic acid testing-negative donors in lung transplantation. Clin Transplant. 2019;33:e13476. https://doi.org/10.1111/ctr.13476.
Woolley AE, Piechura LM, Goldberg HJ, et al. The impact of hepatitis C viremic donor lung allograft characteristics on post-transplantation outcomes. Ann Cardiothorac Surg. 2020;9:42–8. https://doi.org/10.21037/acs.2020.01.03.
Cypel M, Levvey B, Van Raemdonck D, et al. International Society for Heart and Lung Transplantation donation after circulatory death registry report. J Heart Lung Transplant. 2015;34:1278–82.
Smits JM, van der Bij W, Van Raemdonck D, et al. Defining an extended criteria donor lung: an empirical approach based on the Eurotransplant experience. Transpl Int. 2011;24:393–400. https://doi.org/10.1111/j.1432-2277.2010.01207.x.
Oto T, Levvey BJ, Whitford H, et al. Feasibility and utility of a lung donor score: correlation with early post-transplant outcomes. Ann Thorac Surg. 2007;83:257–63. https://doi.org/10.1016/j.athoracsur.2006.07.040.
Whited WM, Trivedi JR, van Berkel VH, Fox MP. Objective donor scoring system for lung transplantation. Ann Thorac Surg. 2019;107:425–9. https://doi.org/10.1016/j.athoracsur.2018.08.034.
Shigemura N, Cordova F, Criner G, Toyoda Y. Current precautions and future directions in lung transplantation during the COVID-19 pandemic - a single center cohort study. Transpl Int. 2020;33:1453–7. https://doi.org/10.1111/tri.13694.
Summary of Current Evidence and Information– Donor SARS-CoV-2 Testing & Organ Recovery from Donors with a History of COVID-19. Organ Procurement and Transplantation Network, US Department of Health and Human Services, https://optn.transplant.hrsa.gov/media/4424/sars-cov-2-summary-of-evidence.pdf Feb 17 2021.
Aslam S, Danziger-Isakov L, Luong M, et al. Guidance from the International Society of Heart and Lung Transplantation regarding the SARS CoV-2 pandemic. Revised December 4, 2020. Available at https://ishlt.org/ishlt/media/documents/SARS-CoV-2_Guidance-for-Cardiothoracic-Transplant-and-VAD-center.pdf. Accessed 25 Jan 2021.
Galvan NTN, Moreno NF, Garza JE, et al. Donor and transplant candidate selection for solid organ transplantation during the COVID-19 pandemic. Am J Transplant. 2020;20:3113–22.
Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2017 annual data report: lung. Am J Transplant. 2019;19:404–84.
De Meester J, Smits JM, Persijn GG, Haverich A. Listing for lung transplantation: life expectancy and transplant effect, stratified by type of end-stage lung disease, the Eurotransplant experience. J Heart Lung Transplant. 2001;20:518–24. https://doi.org/10.1016/s1053-2498(01)00241-8.
Smith S, Trivedi JR, Fox M, Van Berkel VH. Donor Lung Utilization for Transplantation in the United States. J Heart Lung Transplant. 2020;39:S374-S. https://doi.org/10.1016/j.healun.2020.01.471.
Somers J, Ruttens D, Stanzi A, et al. Extended criteria donors; a safe way to expand the lung donor pool? J Heart Lung Transplant. 2013:32:S155. https://doi.org/10.1016/j.healun.2013.01.358.
Zych B, Garcia Saez D, Sabashnikov A, et al. Lung transplantation from donors outside standard acceptability criteria–are they really marginal? Transpl Int. 2014;27:1183–91. https://doi.org/10.1111/tri.12410.
Sommer W, Ius F, Muller C, et al. Extended criteria donor lungs do not impact recipient outcomes in pediatric transplantation. J Heart Lung Transplant. 2019;38:560–9. https://doi.org/10.1016/j.healun.2019.02.012.
Russo MJ, Worku B, Iribarne A, et al. Does lung allocation score maximize survival benefit from lung transplantation? J Thorac Cardiovasc Surg. 2011;141:1270–7. https://doi.org/10.1016/j.jtcvs.2010.12.028.
Mallory GB Jr. Donor-recipient lung size mismatch: a provocative data set. J Heart Lung Transplant. 2013;32:1163–4. https://doi.org/10.1016/j.healun.2013.10.011.
Reitz BA, Wallwork JL, Hunt SA, et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med. 1982;306:557–64. https://doi.org/10.1056/NEJM198203113061001.
Goldstein R, Bolman R, Cooper J, et al. Lung transplantation: report of the ATS Workshop on Lung Transplantation. Am Rev Respir Dis. 1993;147:772–6.
Patterson GA, Cooper JD, Dark JH, Jones MT. Experimental and clinical double lung transplantation. J Thorac Cardiovasc Surg. 1988;95:70–4.
Eberlein M, Arnaoutakis GJ, Yarmus L, et al. The effect of lung size mismatch on complications and resource utilization after bilateral lung transplantation. J Heart Lung Transplant. 2012;31:492–500. https://doi.org/10.1016/j.healun.2011.12.009.
Eberlein M, Reed RM, Bolukbas S, et al. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant. 2015;34:233–40. https://doi.org/10.1016/j.healun.2014.09.030.
Eberlein M, Diehl E, Bolukbas S, Merlo CA, Reed RM. An oversized allograft is associated with improved survival after lung transplantation for idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. 2013;32:1172–8. https://doi.org/10.1016/j.healun.2013.06.011.
Dezube R, Arnaoutakis GJ, Reed RM, et al. The effect of lung-size mismatch on mechanical ventilation tidal volumes after bilateral lung transplantation. Interact Cardiovasc Thorac Surg. 2013;16:275–81. https://doi.org/10.1093/icvts/ivs493.
Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8:492–506. https://doi.org/10.1183/09031936.95.08030492.
Eberlein M, Permutt S, Chahla MF, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest. 2012;141:451–60. https://doi.org/10.1378/chest.11-0767.
Barnard JB, Davies O, Curry P, et al. Size matching in lung transplantation: an evidence-based review. J Heart Lung Transplant. 2013;32:849–60. https://doi.org/10.1016/j.healun.2013.07.002.
Eberlein M, Reed RM, Maidaa M, et al. Donor-recipient size matching and survival after lung transplantation. A cohort study. Ann Am Thorac Soc. 2013;10:418–25. https://doi.org/10.1513/AnnalsATS.201301-008OC.
Kilburn KH, Warshaw RH, Thornton JC, Thornton K, Miller A. Predictive equations for total lung capacity and residual volume calculated from radiographs in a random sample of the Michigan population. Thorax. 1992;47:519–23. https://doi.org/10.1136/thx.47.7.519.
Mason DP, Batizy LH, Wu J et al. Matching donor to recipient in lung transplantation: How much does size matter? J Thorac Cardiovasc Surg. 2009;137:1234–40 e1. https://doi.org/10.1016/j.jtcvs.2008.10.024.
Sunagawa G, Kehara H, Kashem M, et al. Single lung transplant remains a viable alternative to double lung transplantation for the patients with severe secondary pulmonary hypertension. J Heart Lung Transplant. 2021;40:S71–2. https://doi.org/10.1016/j.healun.2021.01.1916.
Benvenuto LJ, Costa J, Piloni D, et al. Right single lung transplantation or double lung transplantation compared with left single lung transplantation in chronic obstructive pulmonary disease. J Heart Lung Transplant. 2020;39:870–7. https://doi.org/10.1016/j.healun.2020.06.009.
Timofte I, Vesselinov R, Terrin M, et al. Single versus double lung transplantation for Chronic Obstructive Lung Disease (COPD). J Heart Lung Transplant. 2019;38:S224. https://doi.org/10.1016/j.healun.2019.01.550.
Brunsting LA, Lupinetti FM, Cascade PN, et al. Pulmonary function in single lung transplantation for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg. 1994;107:1337–44.
Hayden AM, Scarlett MV, Fox K. Relationship between donor/recipient lung size mismatch and functional outcome in single lung transplantation for COPD. J Transpl Coord. 1996;6:155–8.
Estenne M. Effect of lung transplant and volume reduction surgery on respiratory muscle function. J Appl Physiol (1985). 2009;107:977–86. https://doi.org/10.1152/japplphysiol.91620.2008.
Weill D, Torres F, Hodges TN, Olmos JJ, Zamora MR. Acute native lung hyperinflation is not associated with poor outcomes after single lung transplant for emphysema. J Heart Lung Transplant. 1999;18:1080–7.
Tsagkaropoulos S, Belmans A, Verleden GM, et al. Single-lung transplantation: does side matter? Eur J Cardiothorac Surg. 2011;40:e83-92. https://doi.org/10.1016/j.ejcts.2011.03.011.
Bansal S, Shigemura N. Size matching in lung transplantation: new alternatives to an old problem. Minerva Chir. 2015;70:37–41.
Keeshan BC, Rossano JW, Beck N, et al. Lung transplant waitlist mortality: height as a predictor of poor outcomes. Pediatr Transplant. 2015;19:294–300.
Eberlein M, Permutt S, Brown RH, et al. Supranormal expiratory airflow after bilateral lung transplantation is associated with improved survival. Am J Respir Crit Care Med. 2011;183:79–87. https://doi.org/10.1164/rccm.201004-0593OC.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research involving human participants and/or animals
Not applicable as per institutional ethical committee as the scientific information presented in paper does not fall into category of clinical trial or usage of experimental modalities.
Informed consent
Obtained for de-identified data/image publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mangukia, C., Shigemura, N., Stacey, B. et al. Donor quality assessment and size match in lung transplantation. Indian J Thorac Cardiovasc Surg 37 (Suppl 3), 401–415 (2021). https://doi.org/10.1007/s12055-021-01251-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-021-01251-9