Abstract
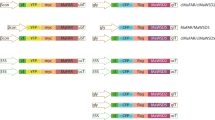
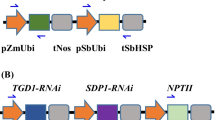
Metabolic engineering of crops is a potential route to economically viable production of polyhydroxyalkanoates (PHAs), biodegradable and renewable alternatives to conventional plastics. In particular, short-chain-length (SCL)/medium-chain-length (MCL) PHA copolymers have attracted commercial interest for their wide range of potential applications. To date, examples of SCL/MCL PHA copolymer production in plant peroxisomes have involved single transgene approaches in transgenic Arabidopsis. We attempted to produce SCL/MCL PHA copolymers using a multigene strategy in peroxisomes of the high biomass food and industrial crop, sugarcane (Saccharum hybrids). Our approach involved peroxisomal targeting of a 3-ketothiolase, acetoacetyl-CoA reductase, enoyl-CoA hydratase and PHA synthase, as well as plastid targeting of a acyl-ACP thioesterase and 3-ketoacyl-ACP synthase to increase peroxisomal β-oxidation flux. Of 143 transgenic sugarcane lines generated by co-bombardment with the six transgenes, six were identified with PHA copolymers at up to 0.015% leaf dry mass, consisting mainly of saturated C4–C16 3-hydroxyalkanoic acids. One line with high acetoacetyl-CoA reductase and low 3-ketothiolase transcript levels had increased 3-hydroxybutyrate content, and acyl-ACP thioesterase and 3-ketoacyl-ACP synthase expression were associated with altered MCL monomer profiles. SCL/MCL PHA copolymer from the highest-yielding line showed a weight-average molecular weight of 111 KDa and polydispersity index of 1.2. Transmission electron microscopy of leaf sections from this line indicated the presence of PHA granules in peroxisomes. This work demonstrates SCL/MCL PHA copolymer biosynthesis in sugarcane peroxisomes and provides a basis for further development of mechanisms for controlling PHA composition in transgenic crop plants.




Similar content being viewed by others
Abbreviations
- ACP:
-
Acyl carrier protein
- DM:
-
Dry mass
- HPLC:
-
High performance liquid chromatography
- PDI:
-
Polydispersity index
- PHA:
-
polyhydroxyalkanoate
- PHB:
-
polyhydroxybutyrate
- SCL:
-
short-chain-length
- MCL:
-
medium-chain-length
- GC-MS:
-
gas chromatography–mass spectrometry
- GPC:
-
gel permeation chromatography
References
Arai Y, Nakashita H, Suzuki Y et al (2002) Synthesis of a novel class of polyhydroxyalkanoates in Arabidopsis peroxisomes, and their use in monitoring short-chain-length intermediates of beta-oxidation. Plant Cell Physiol 43:555–562
Arent S, Pye VE, Henriksen A (2008) Structure and function of plant acyl-CoA oxidases. Plant Physiol Biochem 46:292–301
Birch RG, Bower RS, Elliott AR (2010) Highly efficient, 5′-sequence-specific transgene silencing in a complex polyploid. Trop Plant Biol 3:88–97
Bohlmann GM (2006) Polyhydroxyalkanoate production in crops. In: Bozell JJ (ed) Feedstocks for the future: renewables for the production of chemicals and materials. American Chemical Society Symposium Series 921, vol 921. Oxford University Press, Oxford, pp 253–270
Bohmert K, Balbo I, Kopka J et al (2000) Transgenic Arabidopsis plants can accumulate polyhydroxybutyrate to up to 4% of their fresh weight. Planta 211:841–845
Bower R, Elliott AR, Potier BAM et al (1996) High-efficiency, microprojectile-mediated cotransformation of sugarcane, using visible or selectable markers. Mol Breed 2:239–249
Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes—structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18:675–689
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5:213–218
Ding CM, Cantor CR (2003) A high-throughput gene expression analysis technique using competitive PCR and matrix-assisted laser desorption ionization time-of-flight MS. Proc Natl Acad Sci USA 100:3059–3064
Doi Y, Kitamura S, Abe H (1995) Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromol 28:4822–4828
Froman BE, Edwards PC, Bursch AG et al (2000) ACX3, a novel medium-chain acyl-coenzyme A oxidase from Arabidopsis. Plant Physiol 123:733–741
Goepfert S, Poirier Y (2007) Beta-oxidation in fatty acid degradation and beyond. Curr Opin Plant Biol 10:245–251
Hahn JJ, Eschenlauer AC, Sleytr UB et al (1999) Peroxisomes as sites for synthesis of polyhydroxyalkanoates in transgenic plants. Biotechnol Prog 15:1053–1057
Hazer B, Steinbüchel A (2007) Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. App Microbiol Biotechnol 74:1–12
Holmes PA (1988) Biologically produced PHA polymers and copolymers. In: Bassett DC (ed) Developments in Crystalline Polymers—2. Elsevier Applied Science Publ, London/New York, pp 1–65
Huang AHC, Trelease RN, Moore TS (1983) Plant peroxisomes. Academic, New York
Kato M, Bao HJ, Kang CK et al (1996) Production of a novel copolyester of 3-hydroxybutyric acid and medium chain length 3-hydroxyalkanoic acids by Pseudomonas sp. 61–3 from sugars. App Microbiol Biotechnol 45:363–370
Lee EY, Jendrossek D, Schirmer A et al (1995) Biosynthesis of copolyesters consisting of 3-hydroxybutyric acid and medium-chain length 3-hydroxyalkanoic acids from 1,3-butanediol or from 3-hydroxybutyrate by Pseudomonas sp. A33. App Microbiol Biotechnol 42:901–909
Lee SH, Oh DH, Ahn WS et al (2000) Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by high-cell-density cultivation of Aeromonas hydrophila. Biotechnol Bioeng 67:240–244
Lenz RW, Marchessault RH (2005) Bacterial polyesters: Biosynthesis, biodegradable plastics and biotechnology. Biomacromol 6:1–8
Leonard JM, Slabaugh MB, Knapp SJ (1997) Cuphea wrightii thioesterases have unexpected broad specificities on saturated fatty acids. Plant Mol Biol 34:669–679
Leonard JM, Knapp SJ, Slabaugh MB (1998) A Cuphea beta-ketoacyl-ACP synthase shifts the synthesis of fatty acids towards shorter chains in Arabidopsis seeds expressing Cuphea FatB thioesterases. Plant J 13:621–628
Liebergesell M, Fallis P, Dong J et al (2002) Polyhydroxyalkanoate synthase genes. US Patent 6,475,734,
Matsumoto K, Nagao R, Murata T et al (2005) Enhancement of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production in the transgenic Arabidopsis thaliana by the in vitro evolved highly active mutants of polyhydroxyalkanoate (PHA) synthase from Aeromonas caviae. Biomacromol 6:2126–2130
Matsumoto K, Arai Y, Nagao R et al (2006) Synthesis of short-chain-length/medium-chain-length polyhydroxyalkanoate (PHA) copolymers in peroxisome of the transgenic Arabidopsis thaliana harboring the PHA synthase gene from Pseudomonas sp 61–3. J Polymers Env 14:369–374
Matsumoto K, Murata T, Nagao R et al (2009) Production of short-chain-length/medium-chain-length polyhydroxyalkanoate (PHA) copolymer in the plastid of Arabidopsis thaliana using an engineered 3-ketoacyl-acyl carrier protein synthase III. Biomacromol 10:686–690
McQualter RB, Fong Chong B, Baker A et al (2005) Initial evaluation of sugarcane as a production platform for p-hydroxybenzoic acid. Plant Biotechnol J 3:29–41
Mittendorf V, Robertson EJ, Leech RM et al (1998) Synthesis of medium-chain-length polyhydroxyalkanoates in Arabidopsis thaliana using intermediates of peroxisomal fatty acid beta-oxidation. Proc Natl Acad Sci USA 95:13397–13402
Mittendorf V, Bongcam V, Allenbach L et al (1999) Polyhydroxyalkanoate synthesis in transgenic plants as a new tool to study carbon flow through beta-oxidation. Plant J 20:45–55
Mudge SR, Osabe K, Casu RE et al (2009) Efficient silencing of reporter transgenes coupled to known functional promoters in sugarcane, a highly polyploid crop species. Planta 229:549–558
Nakashita H, Arai Y, Yoshioka K et al (1999) Production of biodegradable polyester by a transgenic tobacco. Biosci Biotechnol Biochem 63:870–874
Noda I, Bond EB, Green PR et al (2005a) Preparation, properties, and utilization of biobased biodegradable NodaxTM copolymers. In: Cheng HN, Gross RA (eds) Polymer Biocatalysis and Biomaterials. American Chemical Society Symposium Series, vol 900. Oxford University Press, Oxford, pp 280–291
Noda I, Green PR, Satkowski MM et al (2005b) Preparation and properties of a novel class of polyhydroxyalkanoate copolymers. Biomacromol 6:580–586
Nomura CT, Taguchi S (2007) PHA synthase engineering toward superbiocatalysts for custom-made biopolymers. App Microbiol Biotechnol 73:969–979
Oeth P, Correll D, Jurinke C (2004) Multiplexed gene expression analysis using competitive PCR and MassARRAYTM. In: Sequenom® Application Notes. Sequenom® Inc. http://www.sequenom.com/Assets/pdfs/appnotes/Multiplexing_for_Gene_Expression_Analysis.pdf Cited 30 June 2008
Petrasovits LA, Purnell MP, Nielsen LK et al (2007) Production of polyhydroxybutyrate in sugarcane. Plant Biotechnol J 5:162–172
Philip S, Keshavarz T, Roy I (2007) Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chemical Technol Biotechnol 82:233–247
Poirier Y, Nawrath C, Somerville C (1995a) Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Biotechnol 13:142–150
Poirier Y, Somerville C, Schechtman LA et al (1995b) Synthesis of high-molecular-weight poly([R]-(−)-3-hydroxybutyrate) in transgenic Arabidopsis thaliana plant cells. Int J Biol Macromol 17:7–12
Poirier Y, Ventre G, Caldelari D (1999) Increased flow of fatty acids toward beta-oxidation in developing seeds of Arabidopsis deficient in diacylglycerol acyltransferase activity or synthesizing medium-chain-length fatty acids. Plant Physiol 121:1359–1366
Poirier Y, Gruys KJ (2005) Production of polyhydroxyalkanoates in transgenic plants. In: Doi Y, Steinbüchel A (eds) Polyesters I: biological systems and biotechnological production. Biopolymers, vol 3a. Wiley-VCH, Weinheim, pp 281–316
Purnell MP, Petrasovits LA, Nielsen LK et al (2007) Spatio-temporal characterization of polyhydroxybutyrate accumulation in sugarcane. Plant Biotechnol J 5:173–184
Rehm BHA (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33
Romano A, van der Plas LHW, Witholt B et al (2005) Expression of poly-3-(R)-hydroxyalkanoate (PHA) polymerase and acyl-CoA-transacylase in plastids of transgenic potato leads to the synthesis of a hydrophobic polymer, presumably medium-chain-length PHAs. Planta 220:455–464
Satkowski MM, Melik DH, Autran J-P et al (2001) Physical and processing properties of polyhydroxyalkanoate copolymers. In: Doi Y, Steinbüchel A (eds) Polyesters II: properties and chemical synthesis. Biopolymers, vol 3b. Wiley VCH, Weinheim, pp 231–264
Slabaugh MB, Leonard JM, Knapp SJ (1998) Condensing enzymes from Cuphea wrightii associated with medium chain fatty acid biosynthesis. Plant J 13:611–620
Steinbüchel A, Valentin HE (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Micro Lett 128:219–228
Steinbüchel A (2001) Perspectives for biotechnological production and utilization of biopolymers: metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example. Macromol Biosci 1:1–24
Steinbüchel A, Hein S (2001) Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. In: Scheper T (ed) Advances in biochemical engineering/biotechnology 71. Springer, Heidelberg, pp 81–123
Tilbrook K, Gnanasambandam A, Schenk PM et al (2010) Efficient targeting of polyhydroxybutyrate biosynthetic enzymes to plant peroxisomes requires more than three amino acids in the carboxyl-terminal signal. J Plant Physiol 167:329–332
Tilbrook K, Gebbie L, Schenk PM et al (2011) Peroxisomal polyhydroxyalkanoate biosynthesis is a promising strategy for bioplastic production in high biomass crops. Plant Biotechnol J (In press). doi:10.1111/j.1467-7652.2011.00600.x
Tsuge T, Taguchi K, Taguchi S et al (2003) Molecular characterization and properties of (R)-specific enoyl-CoA hydratases from Pseudomonas aeruginosa: metabolic tools for synthesis of polyhydroxyalkanoates via fatty acid beta-oxidation. Int J Biol Macromol 31:195–205
van Beilen JB, Poirier Y (2008) Production of renewable polymers from crop plants. Plant J 54:684–701
Vandesompele J, De Preter K, Pattyn F et al (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–12
Volokita M (1991) The carboxy-terminal end of glycolate oxidase directs a foreign protein into tobacco leaf peroxisomes. Plant J 1:361–366
Wang YH, Wu ZY, Zhang XH et al (2005) Synthesis of medium-chain-length-polyhydroxyalkanoates in tobacco via chloroplast genetic engineering. Chinese Sci Bull 50:1113–1120
Zou XH, Chen GQ (2007) Metabolic engineering for microbial production and applications of copolyesters consisting of 3-hydroxybutyrate and medium-chain-length 3-hydroxyalkanoates. Macromol Biosci 7:174–182
Acknowledgements
This work was funded jointly by the Australian Government through the Co-operative Research Centre for Sugarcane Industry Innovation through Biotechnology (CRCSIIB) and BSES Limited. AG was a recipient of a Smart State Fellowship awarded by the Department of State Development, Trade and Innovation of the Queensland Government. We wish to thank Ms. Liz Burns (BSES Limited) for assistance with initial GC-MS screening; Mr. Niall Masel, BSES Limited, for assistance with GPC analysis; Dr. Deb Stenzel, Queensland University of Technology, and Ms. Kimberley Tilbrook, The University of Queensland, for assistance with transmission electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Robert Birch
Rights and permissions
About this article
Cite this article
Anderson, D.J., Gnanasambandam, A., Mills, E. et al. Synthesis of Short-Chain-Length/Medium-Chain Length Polyhydroxyalkanoate (PHA) Copolymers in Peroxisomes of Transgenic Sugarcane Plants. Tropical Plant Biol. 4, 170–184 (2011). https://doi.org/10.1007/s12042-011-9080-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12042-011-9080-7