Abstract
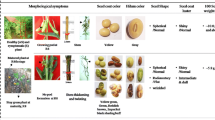
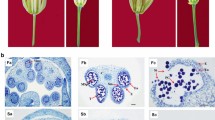
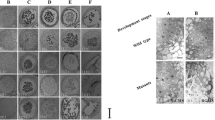
An attempt was made to understand the ‘floral bud distortion’ (FBD), an unexplored disorder prevailing in soybean. Cytological behaviour of floral reproductive organs and in silico characterization of differentially expressed transcript-derived fragments (TDFs) in symptomatic and asymptomatic soybean plants were carried out. Pollens in asymptomatic plants do not have defects in number, size, shape and function. However, in symptomatic plant, pollens were found nonviable, abnormal in shape and with reduced germination ability. Here, we employed a computational approach, exploring invaluable resources. The tissue-specific transcript profile of symptomatic and asymptomatic sources was compared to determine differentially expressed TDFs associated with FBD to improve its basic understanding. A total of 60 decamer primers produced 197 scorable amplicons, ranged 162–1130 bp, of which 171 were monomorphic and 26 were differentially regulated. Reproducible TDFs were sequenced and characterized for their homology analysis, annotation, protein–protein interaction, subcellular localization and their physical mapping. Homology-based annotation of TDFs in soybean revealed presence of two characterized and seven uncharacterized hits. Annotation of characterized sequences showed presence of genes, namely auxin response factor 9 (ARF9) and forkhead-associated (FHA) domain, which are directly involved in plant development through various pathways, such as hormonal regulation, plant morphology, embryogenesis and DNA repair.



Similar content being viewed by others
References
Abel S. and Theologis A. 1996 Early genes and auxin action. Plant Physiol. 111, 9–17.
Ahn E. R., Cho H. K. and Pai H. S. 2013 The forkhead-associated domain 2 (FHA2) in Arabidopsis plays a role in plant fertility by regulating stamen development. Planta 237, 1015–1023.
Altschul S. F., Madden T. L. and Schaffer A. A. 1997 Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402.
Barrow J. R. 1983 Comparisons among pollen viability measurement methods in cotton. Crop Sci. 23, 734–736.
Chen Y. H., Tsai Y. J., Huang J. Z. and Chen F. C. 2005 Transcription analysis of peloric mutants of Phalaenopsis orchids derived from tissue culture. Cell Res. 15, 639–657.
Dafni A. 1992 Pollination ecology: a practical approach. Oxford University Press, Oxford, UK.
Djanaguiraman M., Prasad P. V. V., Boyle D. L. and Schapaugh W. T. 2013 Soybean pollen anatomy, viability and pod set under high temperature stress. J. Agron. Crop. Sci. 199, 171–177.
Finet C., Berne-Dedieu A., Scutt C. P. and Marletaz F. 2013 Evolution of the ARF gene family in land plants: old domains, new tricks. Mol. Biol. Evol. 30, 45–56.
Firmage D. H. and Dafni A. 2001 Field tests for pollen viability; a comparative approach. Acta Hortic. 561, 87–94.
Franceschini A., Szklarczyk D., Frankild S., Kuhn M., Simonovic M., Roth A. and Jensen L. J. 2013 STRING v9. 1: protein–protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 41, D808–D815.
Ghag S. B., Shekhawat U. K. and Ganapathi T. R. 2014 Characterization of Fusarium wilt resistant somaclonal variants of banana cv. Rasthali by cDNA-RAPD. Mol. Biol. Rep. 41, 7929–7935.
Golnaraghi A. R., Shahraeen N., Pourrahim R., Farzadfar S. and Ghasemi A. 2004 Occurrence and relative incidence of viruses infecting soybeans in Iran. Plant Disease. 88, 1069–1074.
Guilfoyle T. J. and Hagen G. 2007 Auxin response factors. Curr. Opin. Plant. Biol. 10, 453–460.
Hagen G. and Guilfoyle T. 2002 Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol. Biol. 49, 373–385.
Heslop-Harrison J. S. 1992 Cytological techniques to assess pollen quality. In Sexual plant reproduction, pp. 41–48. Springer Berlin Heidelberg.
Holdaway-Clarke T. L. and Hepler P. K. 2003 Control of pollen tube growth: role of ion gradients and fluxes. New Phytol. 159, 539–563.
Horton P., Park K. J., Obayashi T., Fujita N., Harada H., Adams-Collier C. J. and Nakai K. 2007 WoLF PSORT: protein localization predictor. Nucleic Acids Res. 35, W585–W587.
Hu Q., Jin Y., Shi H. and Yang W. 2014 GmFLD a soybean homolog of the autonomous pathway gene FLOWERING LOCUS D, promotes flowering in Arabidopsis thaliana. BMC Plant Biol. 14, 263.
Huang F., Xu G., Chi Y., Liu H., Xue Q., Zhao T. et al. 2014 A soybean MADS-box protein modulates floral organ numbers, petal identity and sterility. BMC Plant Biol. 14, 89.
Jadhav P. V., Mane S. S., Nandanwar R. S., Kale P. B., Dudhare M. S., Moharil M. P. and Dani R. G. 2013 Floral bud distortion in soybean and incidence in Central India. Egy. J. Biol. 15, 59–65.
Johri B. M. and Vasil I. K. 1961 Physiology of Pollen. Bot. Rev. 27(3), 318–381.
Kawar P. G., Pagariya M. C., Dixit G. B. and Prasad D. T. 2010 Identification and isolation of SCGS phytoplasma-specific fragments by riboprofiling and development of specific diagnostic tool. J. Plant Biochem. Biot. 19, 185–194.
Knox R. B. 1984 Pollen-pistil interactions. In: Cellular interactions, pp. 508–608. Springer Berlin Heidelberg.
Koti S., Reddy K. R., Reddy V. R. and Zhao D. 2005 Interactive effects of carbon dioxide, temperature, and ultraviolet-B radiation on soybean (Glycine max L.) flower and pollen morphology, pollen production, germination and tube lengths. J. Exp. Bot. 56, 725–736.
Lee I. M., Bottner-Parker K. D., Zhao Y., Villalobos W. and Moreira L. 2011 ‘Candidatus Phytoplasma costaricanum’ a novel phytoplasma associated with an emerging disease in soybean (Glycine max). Int. J. Syst. Evol. Micr. 61, 2822–2826.
Li J., Lee G. L., Doren S. R. V. and Walker J. C. 2000 The FHA domain mediates phosphoprotein interactions. J. Cell Sci. 113, 4143–4149.
Ling H. Y., Edwards A. M., Gantier M. P., DeBoer K. D., Neale A. D., Hamill J. D. and Walmsley A. M. 2012 An interspecific Nicotiana hybrid as a useful and cost-effective platform for production of animal vaccines. PLoS One 7, e35688.
Mahajan A., Yuan C., Pike B. L., Heierhorst J., Chang C. F. and Tsai M. D. 2005 FHA domain-ligand interactions: importance of integrating chemical and biological approaches. J. Am. Chem. Soc. 127, 14572–14573.
Marr D. L. and Marshall M. L. 2006 The role of fungal pathogens in flower size and seed mass variation in three species of Hydrophyllum (Hydrophyllaceae). Am. J. Bot. 93, 389–398.
McInnis S. M., Emery D. C., Porter R., Desikan R., Hancock J. T. and Hiscock S. J. 2006 The role of stigma peroxidases in flowering plants: insights from further characterization of a stigma-specific peroxidase (SSP) from Senecio squalidus (Asteraceae). J. Exp. Bot. 57, 1835–1846.
Nayyar H., Bains T. and Kumar S. 2005 Low temperature induced floral abortion in chickpea: relationship to abscisic acid and cryoprotectants in reproductive organs. Env. Exp. Bot. 53, 39–47.
Nimbalkar S. B., Harsulkar A. M., Giri A. P., Sainani M. N., Franceschi V. and Gupta V. S. 2006 Differentially expressed gene transcripts in roots of resistant and susceptible chickpea plant (Cicer arietinum) upon Fusarium oxysporum infection. Physiol. Mol. Plant. Pathol. 668, 176–188.
Pagariya M. C., Harikrishnan M., Kulkarni P. A., Devarumath R. M. and Kawar P. G. 2011 Physio-biochemical analysis and transcript profiling of Saccharum officinarum L. submitted to salt stress. Acta Physiol. Planta 33, 1411–1424.
Phanchaisri B., Samsang N., Yu L., Singkarat S. and Anuntalabhochai S. 2012 Expression of OsSPY and 14-3-3 genes involved in plant height variations of ion-beam-induced KDML 105 rice mutants. Mutat. Res. Fund. Mol. Mech. Mut. 734, 56–61.
Pracros P., Renaudin J., Eveillard S., Mouras A. and Hernould M. 2006 Tomato flower abnormalities induced by stolburphytoplasma infection are associated with changes of expression of floral development genes. Mol. Plant Microbe. Interact. 19, 62–68.
Silva N., Mendes-Bonato A. B., Sales J. G. C. and Pagliarini M. S 2011 Meiotic behaviour and pollen viability in Moringa oleifera (Moringaceae) cultivated in southern Brazil. Genet. Mol. Res. 10, 1728–1732.
Singh A. K. and Bhatt B. P. 2013 Occurrence of phytoplasma phyllody and witches’ broom disease of Faba bean in Bihar. J. Env. Bio. 34, 837–840.
Sitbon F. and Perrot-Rechenmann C. 1997 Expression of auxin-regulated genes. Physiol. Plant. 100, 443–455.
Soares-Cavalcanti N. M., Belarmino L. C., Kido E. A., Pandolfi V., Marcelino-Guimarães F. C., Rodrigues F. A. et al. 2012 Overall picture of expressed heat shock factors in Glycine max, Lotus japonicus and Medicago truncatula. Genet. Mol. Biol. 35, 247–259.
Srinivasan A., Saxena N. P. and Johansen C. 1999 Cold tolerance during early reproductive growth of chickpea (Cicer arietinum L.), genetic variation in gamete development and function. Field Crops Res. 60, 209–222.
Subekti N. A. 2008 The effects of disease on plant reproduction as basis for breeding for disease resistance. In Proceedings of the scientific seminar and annual meeting of the regional commissioner PEI PFI XIX South Sulawesi 5 November pp. 167–180. Komisariat Daerah Sulawesi Selatan, Indonesia.
Sugano J., Melzer M., Pant A., Radovich T., Fukuda S. and Migita S. 2011 Field evaluations of Tomato Yellow Leaf Curl Virus Resistant Varieties for Commercial Production. College of Tropical Agriculture and Human resources. PD-78. http://scholarspace.manoa.hawaii.edu/bitstream/10125/33260/1/PD-78.pdf.
Szklarczyk D., Franceschini A., Wyder S., Forslund K., Heller D., Huerta-Cepas J. et al. 2014 STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 1, 1–6.
Tiwari S. B., Hagen G. and Guilfoyle T. 2003 The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 15, 533–543.
Tiwari S. B., Wang X. J., Hagen G. and Guilfoyle T. J. 2001 Aux/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13, 2809– 2822.
Ulmasov T., Hagen G. and Guilfoyle T. J. 1997a ARF1, a transcription factor that binds to auxin response elements. Science 276, 1865–1868.
Ulmasov T., Murfett J., Hagen G. and Guilfoyle T. J. 1997b Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9, 1963–1971.
Ulmasov T., Hagen G. and Guilfoyle T. J. 1999 Dimerization and DNA binding of auxin response factors. Plant J. 19, 309–319.
Wang D., Pei K., Fu Y., Sun Z., Li S., Liu H. et al. 2007 Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 394, 13–24.
Wang J. W., Wang L. J., Mao Y. B., Cai W. J., Xue H. W. and Chen X. Y. 2005a Control of root cap formation by microRNA-targeted Auxin Response Factors in Arabidopsis. The Plant Cell 17, 2204–2216.
Wang M., Xu X., Zhang X., Sun S., Wu C., Hou W. et al. 2015 Functional analysis of GmCPDs and investigation of their roles in flowering. PLoS One 10, e0118476.
Wang S., Tiwari S. B., Hagen G. and Guilfoyle T. J. 2005b AUXIN RESPONSE FACTOR7 restores the expression of auxin-responsive genes in mutant Arabidopsis leaf mesophy11 protoplasts. Plant Cell 17, 1979–1993.
Wang Z., Gerstein M. and Snyder M. 2009 RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63.
Xu F., Meng T., Li P., Yu Y., Cui Y., Wang Y. et al. 2011 A soybean dual-specificity kinase, GmSARK, and its Arabidopsis homolog, AtSARK, regulate leaf senescence through synergistic actions of auxin and ethylene. Plant Physiol. 157, 2131–2153.
Yu B., Bi L., Zheng B., Ji L., Chevalier D., Agarwal M. et al. 2008 The FHA domain proteins DAWDLE in Arabidopsis and SNIP1 in humans act in small RNA biogenesis. Proc. Natl. Acad. Sci. USA 105, 10073–10078.
Acknowledgements
We sincerely thank the Department of Atomic Energy (DAE), Board of Research in Nuclear Sciences (BRNS), Government of India, Mumbai, India for providing financial support (grant no. 2013/37B/44/BRNS/1904).
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Jitendra P. Khurana
Kale P. B., Jadhav P. V., Wakekar R. S., Moharil M. P., Deshmukh A. G., Dudhare M. S., Nandanwar R. S., Mane S. S., Manjaya J. G. and Dani R. G. 2016 Cytological behaviour of floral organs and in silico characterization of differentially expressed transcript-derived fragments associated with ‘floral bud distortion’ in soybean. J. Genet. 95, xx–xx
Rights and permissions
About this article
Cite this article
KALE, P.B., JADHAV, P.V., WAKEKAR, R.S. et al. Cytological behaviour of floral organs and in silico characterization of differentially expressed transcript-derived fragments associated with ‘floral bud distortion’ in soybean. J Genet 95, 787–799 (2016). https://doi.org/10.1007/s12041-016-0693-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-016-0693-3