Abstract
This review offers a comprehensive discussion on literature concerning the development of catalytic protocols for the dearomative hydroboration of heteroaromatic compounds. The importance of selective dearomatization of heteroarenes, their remarkable applications along with the development of different catalytic methods and their synthetic scopes are emphasized.
Graphic abstract
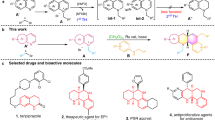
The dearomatization/aromatization process of pyridine motif enables dihydropyridine (DHP) derivatives to act as a key intermediate in deriving biologically active species, pharmaceuticals, organocatalyst and sophisticated reducing agents. This review offers a complete overview of recent development of catalytic protocols for the dearomative hydroboration of heteroaromatic compounds.


























Similar content being viewed by others
References
(a) Pollak N, Dölle C and Ziegler M 2007 The power to reduce: Pyridine nucleotides–small molecules with a multitude of functions Biochem. J. 402 205; (b) Eisner U and Kuthan J 1972 Chemistry of dihydropyridines Chem. Rev. 72 1; (c) Stout evidence and Meyers A I 1982 Recent advances in the chemistry of dihydropyridines Chem. Rev. 82 223; (d) Sliwa W 1986 The reactivity of N-substituted pyridinium salts Heterocycles 24 181; (e) Lavilla R J 2002 Recent developments in the chemistry of dihydropyridines Chem. Soc. Perkin Trans. 1 1141
(a) Rueping M, Dufour J and Schoepke F R 2011 Advances in catalytic metal-free reductions: from bio-inspired concepts to applications in the organocatalytic synthesis of pharmaceuticals and natural products Green Chem. 13 1084; (b) Ouellet S G, Walji A M and Macmillan D W C 2007 Enantioselective organocatalytic transfer hydrogenation reactions using Hantzsch esters Acc. Chem. Res. 40 1327
Edraki N, Mehdipour A R, Khoshneviszadeh M and Miri R 2009 Dihydropyridines: evaluation of their current and future pharmacological applications Drug Discov. Today 14 1058
Satoh N, Akiba T, Yokoshima S and Fukuyama T 2007 A practical synthesis of (–)-oseltamivir Angew. Chem. Int. Ed. 46 5734
(a) Cozzi P, Carganico G, Fusar D, Grossoni M, Menichincheri M, Pinciroli V, Tonani R, Vaghi F and Salvati P 1993 Imidazol-1-yl and pyridin-3-yl derivatives of 4-phenyl-1,4-dihydropyridines combining Ca2+ antagonism and thromboxane A2 synthase inhibition J. Med. Chem. 36 2964; (b) Kumar P P, Stotz S C, Paramashivappa R, Beedle A M, Zamponi G W and Rao A S 2002 Synthesis and evaluation of a new class of nifedipine analogs with T-type calcium channel blocking activity Mol. Pharmacol. 61 649
Bull J A, Mousseau J J, Pelletier G and Charette A B 2012 Synthesis of pyridine and dihydropyridine derivatives by regio- and stereoselective addition to N-activated pyridines Chem. Rev. 112 2642
(a) Adkins H, Kuick L F, Farlow M and Wojcik B 1934 Hydrogenation of derivatives of pyridine J. Am. Chem. Soc. 56 2425; (b) Freifelder M and Stone G R 1961 Reductions with ruthenium. II. Its use in the hydrogenation of pyridines1 J. Org. Chem. 26 3805; (c) Lunn G and Sansone E B 1986 Facile reduction of pyridines with nickel-aluminum alloy J. Org. Chem. 51 513; (d) Takasaki M, Motoyama Y, Higashi K, Yoon S-H, Mochida I and Nagashima H 2007 Ruthenium nanoparticles on nano-Level - controlled carbon supports as highly effective catalysts for arene hydrogenation Chem. Asian J. 2 1524; (e) Buil M L, Esteruelas M A, Niembro S, Olivan M, Orzechowski L, Pelayo C and Vallribera A 2010 Dehalogenation and hydrogenation of aromatic compounds catalyzed by nanoparticles generated from rhodium bis(imino)pyridine complexes Organometallics 29 4375; (f) Glorius F 2005 Asymmetric hydrogenation of aromatic compounds Org. Biomol. Chem. 3 4171
Fowler, F. W. 1972 Synthesis of 1,2- and 1,4-dihydropyridines J. Org. Chem. 37 1321
(a) Hao L, Harrod J F, Lebuis A-M, Mu Y, Shu R, Samuel E and Woo H-G 1998 Homogeneous catalytic hydrosilylation of pyridines Angew. Chem. Int. Ed. 37 3126; (b) Harrod J F, Shu R, Woo H G and Samuel E 2001 Titanocene(III) catalyzed homogeneous hydrosilyation-hydrogenation of pyridines Can. J. Chem. 79 1075; (c) Gutsulyak D V, van der Est A and Nikonov G I 2011 Facile catalytic hydrosilylation of pyridines Angew. Chem. Int. Ed. 50 1384; (d) Königs C D F, Klare H F T and Ostreich M 2013 Catalytic 1,4-Selective hydrosilylation of pyridines and benzannulated congeners Angew. Chem. Int. Ed. 52 10076
Oshima K, Ohmura T and Suginome M 2011 Palladium-catalyzed regioselective silaboration of pyridines leading to the synthesis of silylated dihydropyridines J. Am. Chem. Soc. 133 7324
Daley E N, Vogels C M, Geier S J, Decken A, Doherty S and Westcott S A 2015 The phosphinoboration reaction Angew. Chem. Int. Ed. 54 2121
Liu H, Khononov M and Eisen M S 2018 Catalytic 1,2-regioselective dearomatization of N-heteroaromatics via a hydroboration ACS Catal. 8 3673
Dudnik A S, Weidner V L, Motta A, Delferro M and Marks T Z 2014 Atom-efficient regioselective 1,2-dearomatization of functionalized pyridines by an earth-abundant organolanthanide catalyst Nat. Chem. 6 1100
Ji P, Sawano T, Lin Z, Urban A, Boures D and Lin W 2016 Cerium-hydride secondary building units in a porous metal–organic framework for catalytic hydroboration and hydrophosphination J. Am. Chem. Soc. 138 14860
Oshima K, OhmuraT and Suginome M 2012 Regioselective synthesis of 1,2-dihydropyridines by rhodium-catalyzed hydroboration of pyridines J. Am. Chem. Soc. 134 3699
Kaithal A, Chatterjee B and Gunanathan C 2016 Ruthenium-catalyzed regioselective 1,4-hydroboration of pyridines Org. Lett. 18 3402
(a) Kaithal A, Chatterjee B and Gunanathan C 2015 Ruthenium catalyzed selective hydroboration of carbonyl compounds Org. Lett. 17 4790; (b) Chatterjee B and Gunanathan C 2014 Ruthenium catalyzed selective hydrosilylation of aldehydes Chem. Commun. 50 888; (c) Kaithal A, Chatterjee B and Gunanathan C 2016 Ruthenium-catalyzed selective hydroboration of nitriles and imines J. Org. Chem. 81 11153; (d) Kisan S, Krishnakumar V and Gunanathan C 2017 Ruthenium-catalyzed anti-Markovnikov selective hydroboration of olefins ACS Catal. 7 5950; (e) Kisan S, Krishnakumar V and Gunanathan C 2018 Ruthenium-catalyzed deoxygenative hydroboration of carboxylic acids ACS Catal. 8 4772
Tamang S R, Singh A, Unruh D K and Findlater M 2018 Nickel-catalyzed regioselective 1,4-hydroboration of N-heteroarenes ACS Catal. 8 6186
(a) Gunanathan C and Milstein D 2011 Metal-ligand cooperation by aromatization- dearomatization: A new paradigm in bond activation and “green” catalysis Acc. Chem. Res. 44 588; (b) Morris R H 2015 Exploiting metal–ligand bifunctional reactions in the design of iron asymmetric hydrogenation catalysts Acc. Chem. Res. 48 1494; (c) Zell T and Milstein D 2015 Hydrogenation and dehydrogenation iron pincer catalysts capable of metal–ligand cooperation by aromatization/dearomatization Acc. Chem. Res. 48 1979
Königs C D F, Klare H F and Oestreich M 2013 Catalytic 1,4-selective hydrosilylation of pyridines and benzannulated congeners Angew. Chem. Int. Ed. 52 10076
Liu J, Chen J-Y, Jia M, Ming B, Jia J, Liao R-Z, Tung C-H and Wang W 2019 Ni–O Cooperation versus nickel(II) hydride in catalytic hydroboration of N-heteroarenes ACS Catal. 9 3849
Zhang F, Song H, Zhuang X, Tung C-H and Wang W 2017 Iron-catalyzed 1,2-selective hydroboration of N-heteroarenes J. Am. Chem. Soc. 139 17775
(a) Stahl T, Muther K, Ohki Y, Tatsumi K and Oestreich M 2013 Catalytic generation of borenium ions by cooperative B–H bond activation: The elusive direct electrophilic borylation of nitrogen heterocycles with pinacolborane J. Am. Chem. Soc. 135 10978; (b) Omann L, Konigs C D F, Klare H F T and Oestreich M 2017 Cooperative catalysis at metal-sulfur bonds Acc. Chem. Res. 50 1258
Lortie J L, Dudding T, Gabidullin B M and Nikonov G I 2017 Zinc-catalyzed hydrosilylation and hydroboration of N-heterocycles ACS Catal. 7 8454
Ji P, Feng X, Veroneau S S, Song Y and Lin W 2017 Trivalent zirconium and hafnium metal–organic frameworks for catalytic 1,4-dearomative additions of pyridines and quinolines J. Am. Chem. Soc. 139 15600
Feng X, Song Y, Chen J S, Li Z, Chen E Y, Kaufmann M, Wang C and Lin W 2019 Cobalt-bridged secondary building units in a titanium metal–organic framework catalyze cascade reduction of N-heteroarenes Chem. Sci. 10 2193
Fan X, Zheng J, Li Z H and Wang H 2015 Organoborane catalyzed regioselective 1,4-hydroboration of pyridines J. Am. Chem. Soc. 137 4916
Keyzer E N, Kang S S, Hanf S and Wright D S 2017 Regioselective 1,4-hydroboration of pyridines catalyzed by an acid-initiated boronium cation Chem. Commun. 53 9434
Rao B, Chong C C and Kinjo R 2018 Metal-Free Regio- and Chemoselective hydroboration of pyridines catalyzed by 1,3,2-diazaphosphenium triflate J. Am. Chem. Soc. 140 652
McGough J S, Cid J and Ingleson M J 2017 Catalytic electrophilic C–H borylation using NHC- boranes and iodine forms C2-, not C3-, borylated indoles Chem. Eur. J. 23 8180
(a) Wang W-B, Lu S-M, Yang P-Y, Han X-W and Zhou Y-G 2003 Highly enantioselective iridium-catalyzed hydrogenation of heteroaromatic compounds, quinolines J. Am. Chem. Soc. 125 10536; (b) Chen F, Surkus A-E, He L, Pohl M-M, Radnik J, Topf C, Junge K and Beller M 2015 Selective catalytic hydrogenation of heteroarenes with N-graphene-modified cobalt nanoparticles (Co3O4–Co/NGr@α-Al2O3) J. Am. Chem. Soc. 37 11718
Yang C-H, Chen X, Li H, Wei W, Yang Z and Chang J 2018 Iodine catalyzed reduction of quinolines under mild reaction conditions Chem. Commun. 54 8622
Intemann J, Lutz M and Harder S 2014 Multinuclear magnesium hydride clusters: selective reduction and catalytic hydroboration of pyridines Organometallics 33 5722
Lemmerz L E, Spaniol T P and Okuda J 2018 1,4-Dihydropyridyl complexes of magnesium: synthesis by pyridine insertion into the magnesium–silicon bond of triphenylsilyls and catalytic pyridine hydrofunctionalization Dalton Trans. 47 12553
Arrowsmith M, Hill M S, Hadlington T, Kociok-Köhn G and Weetman C 2011 Magnesium-catalyzed hydroboration of pyridines Organometallics 30 5556
Rauch M, Ruccolo S and Parkin G 2017 Synthesis, structure, and reactivity of a terminal magnesium hydride compound with a carbatrane motif, [TismPriBenz]MgH: A multifunctional catalyst for hydrosilylation and hydroboration J. Am. Chem. Soc. 139 13264
Jeong E, Heo J, Park S and Chang S 2019 Alkoxide-promoted selective hydroboration of N-heteroarenes: Pivotal roles of in situ generated BH3 in the dearomatization process Chem. Eur. J. 25 1
Liu T, He J and Zhang Y 2019 Regioselective 1, 2-hydroboration of N-heteroarenes by potassium-based catalyst Org. Chem. Front. 6 2749
Acknowledgement
CG thanks SERB New Delhi (EMR/2016/002517), DAE and NISER for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue on 150 years of the Periodic Table
Rights and permissions
About this article
Cite this article
Chatterjee, B., Gunanathan, C. Catalytic dearomative hydroboration of heteroaromatic compounds. J Chem Sci 131, 118 (2019). https://doi.org/10.1007/s12039-019-1710-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-019-1710-x