Abstract
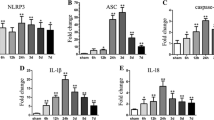
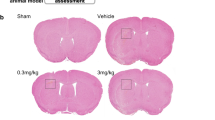
Secondary brain injury (SBI) is a noticeable contributor to the high mortality and morbidity rates associated with intracerebral hemorrhage (ICH), and effective treatment options remain limited. Cystatin C (CysC) emerges as a novel candidate for SBI intervention. The therapeutic effects and underlying mechanisms of CysC in mitigating SBI following ICH were explored in the current research. An in vivo ICH rat model was established by injecting autologous blood into the right caudate nucleus. Western blotting (WB) was utilized to assess the levels of CysC, cathepsin B (CTSB), and the NLRP3 inflammasome. Subsequently, the ICH rat model was treated with exogenous CysC supplementation or CysC knockdown plasmids. Various parameters, including Evans blue (EB) extravasation, brain water content, and neurological function in rats, were examined. RT-qPCR and WB were employed to determine the expression levels of CTSB and the NLRP3 inflammasome. The co-expression of CTSB, CysC, and NLRP3 inflammasome with GFAP, NeuN, and Iba1 was assessed through double-labeled immunofluorescence. The interaction between CysC and CTSB was investigated using double-labeled immunofluorescence and co-immunoprecipitation. The findings revealed an elevation of CysC expression level, particularly at 24 h after ICH. Exogenous CysC supplementation alleviated severe brain edema, neurological deficit scores, and EB extravasation induced by ICH. Conversely, CysC knockdown produced opposite effects. The expression levels of CTSB and the NLRP3 inflammasome were significantly risen following ICH, and exogenous CysC supplement attenuated their expression levels. Double-labeled immunofluorescence illustrated that CysC, CTSB, and the NLRP3 inflammasome were predominantly expressed in microglial cells, and the interaction between CysC and CTSB was evidenced. CysC exhibited potential in ameliorating SBI following ICH via effectively suppressing the activation of the NLRP3 inflammasome mediated by CTSB specifically in microglial cells. These findings underscore the prospective therapeutic efficacy of CysC in the treatment of ICH-induced complications.









Similar content being viewed by others
Data Availability
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
References
Campbell BCV, Khatri P (2020) Stroke. Lancet 396(10244):129–142
Ma Q, Li R, Wang L et al (2021) Temporal trend and attributable risk factors of stroke burden in China, 1990–2019: an analysis for the global burden of disease study 2019. Lancet Public Health 6(12):e897–e906
Magid-Bernstein J, Girard R, Polster S et al (2022) Cerebral hemorrhage: pathophysiology, treatment, and future directions. Circ Res 130(8):1204–1229
Belur PK, Chang JJ, He S et al (2013) Emerging experimental therapies for intracerebral hemorrhage: targeting mechanisms of secondary brain injury. Neurosurg Focus 34(5):E9
Zhu H, Wang Z, Yu J et al (2019) Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol 178:101610
GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021 (10):795-820
Shao Z, Tu S, Shao A (2019) Pathophysiological Mechanisms and potential therapeutic targets in intracerebral hemorrhage. Front Pharmacol 19(10):1079
Li X, Gao X, Zhang W et al (2022) Microglial replacement in the aged brain restricts neuroinflammation following intracerebral hemorrhage. Cell Death Dis 13(1):3
Feng X, Zhan F, Luo D et al (2021) LncRNA 4344 promotes NLRP3-related neuroinflammation and cognitive impairment by targeting miR-138-5p. Brain Behav Immun 98:283–298
Luo Y, Reis C, Chen S (2019) NLRP3 inflammasome in the pathophysiology of hemorrhagic stroke: a review. Curr Neuropharmacol 17(7):582–589
Xiao L, Zheng H, Li J et al (2020) Neuroinflammation mediated by NLRP3 inflammasome after intracerebral hemorrhage and potential therapeutic targets. Mol Neurobiol 57(12):5130–5149
Zhang H, Wang J, Ruan C et al (2022) Co-exposure of chronic stress and alumina nanoparticles aggravates hippocampal microglia pyroptosis by activating cathepsin B/NLRP3 signaling pathway. J Hazard Mater 15(436):129093
Kim JK, Jung HJ, Hyun M et al (2023) Resistance of hypervirulent Klebsiella pneumoniae to cathepsin B-mediated pyroptosis in murine macrophages. Front Immunol 29(14):1207121
Bernstein HG, Kirschke H, Wiederanders B et al (1996) The possible place of cathepsins and cystatins in the puzzle of Alzheimer disease: a review. Mol Chem Neuropathol 27(3):225–247
Jiang Y, Zhang J, Zhang C et al (2020) The role of cystatin C as a proteasome inhibitor in multiple myeloma. Hematology 25(1):457–463
Pérez-González R, Sahoo S, Gauthier SA et al (2019) Neuroprotection mediated by cystatin C-loaded extracellular vesicles. Sci Rep 9(1):11104
Howie AJ, Burnett D, Crocker J (1985) The distribution of cathepsin B in human tissues. J Pathol 145(4):307–314
Hook V, Yoon M, Mosier C et al (2020) Cathepsin B in neurodegeneration of Alzheimer’s disease, traumatic brain injury, and related brain disorders. Biochim Biophys Acta Proteins Proteom 1868(8):140428
Grubb A (1992) Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol 38(Suppl 1):S20–S27
Yang B, Xu J, Chang L et al (2020) Cystatin C improves blood-brain barrier integrity after ischemic brain injury in mice. J Neurochem 153(3):413–425
Liu Y, Li J, Wang Z et al (2014) Attenuation of early brain injury and learning deficits following experimental subarachnoid hemorrhage secondary to cystatin C: possible involvement of the autophagy pathway. Mol Neurobiol 49(2):1043–1054
Wu G, Wang L, Hong Z et al (2011) Effects of minimally invasive procedures for removal of intracranial hematoma on matrix metalloproteinase expression and blood-brain barrier permeability in perihematomal brain tissues. Neurol Res 33(3):300–306
Jiao Y, Ren S, Wang L et al (2023) PPARγ/RAD21 alleviates peripheral secondary brain injury in rat cerebral hemorrhage model through promoting M2 polarization of microglial cells. Int Immunopharmacol 114:10957
Yang B, Zhu J, Miao Z et al (2015) Cystatin C is an independent risk factor and therapeutic target for acute ischemic stroke. Neurotox Res 28(1):1–7
Gan H, Zhang L, Chen H et al (2021) The pivotal role of the NLRC4 inflammasome in neuroinflammation after intracerebral hemorrhage in rats. Exp Mol Med 53(11):1807–1818
Wu G, Sheng F, Wang L et al (2012) The pathophysiological time window study of performing minimally invasive procedures for the intracerebral hematoma evacuation in rabbit. Brain Res 17(1465):57–65
Shi X, Bai H, Wang J et al (2021) Behavioral assessment of sensory, motor, emotion, and cognition in rodent models of intracerebral hemorrhage. Front Neurol 17(12):667511
Chen Y, Chen S, Chang J et al (2021) Perihematomal edema after intracerebral hemorrhage: an update on pathogenesis, risk factors, and therapeutic advances. Front Immunol 19(12):740632
Shao L, Chen S, Ma L (2022) Secondary brain injury by oxidative stress after cerebral hemorrhage: recent advances. Front Cell Neurosci 23(16):853589. https://doi.org/10.3389/fncel.2022.853589
Kaur G, Gauthier SA, Perez-Gonzalez R et al (2018) Cystatin C prevents neuronal loss and behavioral deficits via the endosomal pathway in a mouse model of down syndrome. Neurobiol Dis 120:165–173
Amin F, Khan MS, Bano B (2020) Mammalian cystatin and protagonists in brain diseases. J Biomol Struct Dyn 38(7):2171–2196
Liu H, Shen F, Zhang H et al (2023) Expression and role of cystatin C in hyperthermia-induced brain injury in rats. Math Biosci Eng 20(2):2716–2731
Zhou Y, Dong W, Wang L et al (2023) Lower serum cystatin C level predicts poor functional outcome in patients with hypertensive intracerebral hemorrhage independent of renal function. J Clin Hypertens (Greenwich) 25(1):86–94
Yao D, Li S, Jing J et al (2022) Association of serum cystatin C with cerebral small vessel disease in community-based population. Stroke 53(10):3123–3132
Mathews PM, Levy E (2016) Cystatin C in aging and in Alzheimer’s disease. Ageing Res Rev 32:38–50
Cao B, Luo M, Li J et al (2022) Cerebrospinal fluid cystatin C levels in patients with anti-NMDAR encephalitis and other neurological diseases. J Neuroimmunol 15(369):577900
Fang Z, Deng J, Wu Z et al (2017) Cystatin C is a crucial endogenous protective determinant against stroke. Stroke 48(2):436–444
Martinez-Vargas M, Soto-Nuñez M, Tabla-Ramon E et al (2014) Cystatin C has a dual role in post-traumatic brain injury recovery. Int J Mol Sci 15(4):5807–5820
Nagai A, Ryu JK, Terashima M et al (2005) Neuronal cell death induced by cystatin C in vivo and in cultured human CNS neurons is inhibited with cathepsin B. Brain Res 1066(1–2):120–128
Olsson T, Nygren J, Håkansson K et al (2004) Gene deletion of cystatin C aggravates brain damage following focal ischemia but mitigates the neuronal injury after global ischemia in the mouse. Neuroscience 128(1):65–71
Tizon B, Sahoo S, Yu H et al (2010) Induction of autophagy by cystatin C: a mechanism that protects murine primary cortical neurons and neuronal cell lines. PLoS ONE 5(3):e9819
Xue M, Yong VW (2020) Neuroinflammation in intracerebral haemorrhage: immunotherapies with potential for translation. Lancet Neurol 19(12):1023–1032
Gu L, Sun M, Li R et al (2022) Microglial pyroptosis: therapeutic target in secondary brain injury following intracerebral hemorrhage. Front Cell Neurosci 9(16):971469
Fu J, Wu H (2023) Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu Rev Immunol 26(41):301–316
Zhang J, Liu X, Wan C et al (2020) NLRP3 inflammasome mediates M1 macrophage polarization and IL-1β production in inflammatory root resorption. J Clin Periodontol 47(4):451–460
Song D, Yeh CT, Wang J et al (2022) Perspectives on the mechanism of pyroptosis after intracerebral hemorrhage. Front Immunol. 5(13):989503
Xiao L, Wang M, Shi Y et al (2023) Secondary white matter injury mediated by neuroinflammation after intracerebral hemorrhage and promising therapeutic strategies of targeting the NLRP3 inflammasome. Curr Neuropharmacol 21(3):669–686
Cheng Y, Chen B, Xie W et al (2020) Ghrelin attenuates secondary brain injury following intracerebral hemorrhage by inhibiting NLRP3 inflammasome activation and promoting Nrf2/ARE signaling pathway in mice. Int Immunopharmacol 79:106180
Fu K, Xu W, Lenahan C et al (2023) Autophagy regulates inflammation in intracerebral hemorrhage: Enemy or friend? Front Cell Neurosci 16(16):1036313
Campden RI, Zhang Y (2019) The role of lysosomal cysteine cathepsins in NLRP3 inflammasome activation. Arch Biochem Biophys 30(670):32–42
Liu C, Yao Q, Hu T et al (2022) Cathepsin B deteriorates diabetic cardiomyopathy induced by streptozotocin via promoting NLRP3-mediated pyroptosis. Mol Ther Nucleic Acids 23(30):198–207
Halle A, Hornung V, Petzold GC et al (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9(8):857–865
Liu C, Tang J, Liu S et al (2022) Cathepsin B/NLRP3/GSDMD axis-mediated macrophage pyroptosis induces inflammation and fibrosis in systemic sclerosis. J Dermatol Sci 108(3):127–137
Wang Y, Huang H, He W et al (2021) Association between serum NLRP3 and malignant brain edema in patients with acute ischemic stroke. BMC Neurol 21(1):341
Sun B, Zhou Y, Halabisky B et al (2008) Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer’s disease. Neuron 60(2):247–257
Hook G, Reinheckel T, Ni J et al (2022) Cathepsin B gene knockout improves behavioral deficits and reduces pathology in models of neurologic disorders. Pharmacol Rev 74(3):600–629
Yang D, Han Y, Zhang J et al (2011) Improvement in recovery after experimental intracerebral hemorrhage using a selective cathepsin B and L inhibitor. J Neurosurg 114(4):1110–1116
Biasizzo M, Trstenjak-Prebanda M, Dolinar K et al (2021) Cystatin C deficiency increases LPS-induced sepsis and NLRP3 Inflammasome activation in mice. Cells 10(8):2071
Acknowledgements
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
Funding
This work was supported by The Cultivation Program of the Natural Science Foundation of China (gyfynsfc [gyfynsfc2022]-1, the Cultivation Program of the Natural Science Foundation of China, gyfynsfc[2023]-30, and the Science and Technology Foundation of Guizhou Provincial Health Committee (grant no. gzwkj2023-263).
Author information
Authors and Affiliations
Contributions
Yongfang Zhou: experimental procedures and manuscript preparation. Wentao Dong: experimental procedures. Likun Wang: experimental procedures. Siying Ren: experimental procedures. Weiqing Wei: experimental procedures. Guofeng Wu: study design and manuscript preparation.
Corresponding author
Ethics declarations
Ethics Approval
Guizhou Medical University’s Institutional Animal Conservation and Utilization Committee reviewed the animal study and approved it.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Dong, W., Wang, L. et al. Cystatin C Attenuates Perihematomal Secondary Brain Injury by Inhibiting the Cathepsin B/NLRP3 Signaling Pathway in a Rat Model of Intracerebral Hemorrhage. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04195-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04195-4