Abstract
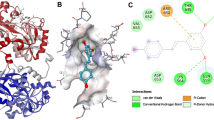
In present times, a switch from chemical molecules towards natural products is taking place, and the latter is being increasingly explored in the management of diseases due to their broad range of therapeutic potential. Consumption of coffee is thought to reduce Alzheimer’s disease (AD); however, the mechanism is still unexplored. Primarily, it is thought that components of coffee are the key players in making it a neuroprotectant. Caffeic acid (CA) is found in high quantities in coffee; thus, it is being increasingly explored to decipher its neuroprotection by various mechanisms. Iron is a toxic element in a free form capable of causing oxidative damage and ultimately contributing to the pathogenesis of AD. Thus, maintaining the proper iron levels is vital and human transferrin (Htf), a glycoprotein, is a key player in this aspect. In this work, we explored the binding mechanism of CA with Htf at the atomistic level, employing molecular docking and extensive molecular dynamics simulation (MD) approaches coupled with spectroscopic techniques in a bid to decipher the mode of interaction of CA with Htf. Molecular docking results demonstrated a strong binding affinity between CA and Htf. Furthermore, MD study highlighted the Htf-CA complex’s stability and the ligand’s minimal impact on Htf’s overall structure. In silico approaches were further backed up by experimental approaches. Strong binding of CA with Htf was ascertained by UV–visible and fluorescence spectroscopy observations. Together, the study provides a comprehensive understanding of the Htf-CA interaction, adding to the knowledge of the use of CA in the treatment of AD, thereby adding another feather to its already known neuroprotective role.







Similar content being viewed by others
Data Availability
The information supporting this study is available in this article.
References
Sun W, Shahrajabian MH (2023) Therapeutic potential of phenolic compounds in medicinal plants—natural health products for human health. Molecules 28(4):1845
Shahwan M, Alhumaydhi F, Ashraf GM, Hasan PM, Shamsi A (2022) Role of polyphenols in combating type 2 diabetes and insulin resistance. Int J Biol Macromol 206:567–579
Andrade S, Ramalho MJ, Loureiro JA, Pereira MdC (2019) Natural compounds for Alzheimer’s disease therapy: a systematic review of preclinical and clinical studies. Int J Mol Sci 20(9):2313
Nan Y, Su H, Zhou B, Liu S (2023) The function of natural compounds in important anticancer mechanisms. Front Oncol 12:1049888
Shamsi A, Anwar S, Mohammad T, Shahwan M, Hassan MI, Islam A (2022) Therapeutic potential of polyphenols in Alzheimer’s therapy: broad-spectrum and minimal side effects as key aspects. In: Autism spectrum disorder and Alzheimer’s disease: advances in research. Springer, pp 111–133
García-Cañas V, Simó C, León C, Cifuentes A (2010) Advances in nutrigenomics research: novel and future analytical approaches to investigate the biological activity of natural compounds and food functions. J Pharm Biomed Anal 51(2):290–304
Yong YY, Dykes GA, Choo WS (2019) Biofilm formation by staphylococci in health-related environments and recent reports on their control using natural compounds. Crit Rev Microbiol 45(2):201–222
Kim JW, Byun MS, Yi D, Lee JH, Jeon SY, Jung G, Lee HN, Sohn BK et al (2019) Coffee intake and decreased amyloid pathology in human brain. Transl Psychiatry 9(1):270
Mancini RS, Wang Y, Weaver DF (2018) Phenylindanes in brewed coffee inhibit amyloid-beta and tau aggregation. Front Neurosci 12:735
El-Seedi HR, El-Said AM, Khalifa SA, Goransson U, Bohlin L, Borg-Karlson A-K, Verpoorte R (2012) Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J Agric Food Chem 60(44):10877–10895
Andrade S, Loureiro JA, Pereira MC (2021) Caffeic acid for the prevention and treatment of Alzheimer’s disease: the effect of lipid membranes on the inhibition of aggregation and disruption of Aβ fibrils. Int J Biol Macromol 190:853–861
Shamsi A, Mohammad T, Khan MS, Shahwan M, Husain FM, Rehman MT, Hassan MI, Ahmad F et al (2019) Unraveling binding mechanism of Alzheimer’s drug rivastigmine tartrate with human transferrin: molecular docking and multi-spectroscopic approach towards neurodegenerative diseases. Biomolecules 9(9):495
Thompson K, Menzies S, Muckenthaler M, Torti FM, Wood T, Torti SV, Hentze MW, Beard J et al (2003) Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J Neurosci Res 71(1):46–63
Stankiewicz J, Panter SS, Neema M, Arora A, Batt CE, Bakshi R (2007) Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics 4(3):371–386
Lu C-D, Ma J-K, Luo Z-Y, Tai Q-X, Wang P, Guan P-P (2018) Transferrin is responsible for mediating the effects of iron ions on the regulation of anterior pharynx-defective-1α/β and presenilin 1 expression via PGE2 and PGD2 at the early stage of Alzheimer’s disease. Aging (Albany NY) 10(11):3117
Guan J, Wang P, Lu L, Zhao G (2020) Association of plasma transferrin with cognitive decline in patients with mild cognitive impairment and Alzheimer’s disease. Front Aging Neurosci 12:38
Goodman L (1953) Alzheimer’s disease: a clinico-pathologic analysis of twenty-three cases with a theory on pathogenesis. J Nerv Ment Dis 118(2):97–130
Xue B, DasGupta D, Alam M, Khan MS, Wang S, Shamsi A, Islam A, Hassan MI (2022) Investigating binding mechanism of thymoquinone to human transferrin, targeting Alzheimer’s disease therapy. J Cell Biochem 123(8):1381–1393
Shamsi A, Ahmed A, Khan MS, Al Shahwan M, Husain FM, Bano B (2020) Understanding the binding between rosmarinic acid and serum albumin: In vitro and in silico insight. J Mol Liq 311:113348
Shamsi A, Mohammad T, Anwar S, Alajmi MF, Hussain A, Hassan MI, Ahmad F, Islam A (2020) Probing the interaction of rivastigmine tartrate, an important Alzheimer’s drug, with serum albumin: attempting treatment of Alzheimer’s disease. Int J Biol Macromol 148:533–542
Shamsi A, Mohammad T, Anwar S, Nasreen K, Hassan MI, Ahmad F, Islam A (2020) Insight into the binding of PEG-400 with eye protein alpha-crystallin: multi spectroscopic and computational approach: possible therapeutics targeting eye diseases. J Biomol Struct Dynam 1–11
Huey R, Morris GM, Forli S (2012) Using AutoDock 4 and AutoDock vina with AutoDockTools: a tutorial. The Scripps Research Institute Molecular Graphics Laboratory 10550:92037
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
DeLano WL (2002) Pymol: an open-source molecular graphics tool. CCP4 Newsl Protein Crystallogr 40 (1):82–92
Biovia DS (2017) Discovery studio visualizer. San Diego, CA, USA 936
Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ (2005) GROMACS: fast, flexible, and free. J Comput Chem 26(16):1701–1718
Wu Y, Tepper HL, Voth GA (2006) Flexible simple point-charge water model with improved liquid-state properties. J Chem Phys 124(2):024503
Turner P (2005) XMGRACE, Version 5.1. 19. Center for Coastal and Land-Margin Research, Oregon Graduate Institute of Science and Technology, Beaverton, OR 2
Noinaj N, Easley NC, Oke M, Mizuno N, Gumbart J, Boura E, Steere AN, Zak O et al (2012) Structural basis for iron piracy by pathogenic Neisseria. Nature 483(7387):53–58
Calmettes C, Alcantara J, Yu R-H, Schryvers AB, Moraes TF (2012) The structural basis of transferrin sequestration by transferrin-binding protein B. Nat Struct Mol Biol 19(3):358–360
Salsbury FR Jr (2010) Molecular dynamics simulations of protein dynamics and their relevance to drug discovery. Curr Opin Pharmacol 10(6):738–744
Durrant JD, McCammon JA (2011) Molecular dynamics simulations and drug discovery. BMC Biol 9(1):1–9
Mohammad T, Shamsi A, Anwar S, Umair M, Hussain A, Rehman MT, AlAjmi MF, Islam A et al (2020) Identification of high-affinity inhibitors of SARS-CoV-2 main protease: towards the development of effective COVID-19 therapy. Virus Res 288:198102
Naqvi AA, Mohammad T, Hasan GM, Hassan M (2018) Advancements in docking and molecular dynamics simulations towards ligand-receptor interactions and structure-function relationships. Curr Top Med Chem 18(20):1755–1768
Durham E, Dorr B, Woetzel N, Staritzbichler R, Meiler J (2009) Solvent accessible surface area approximations for rapid and accurate protein structure prediction. J Mol Model 15(9):1093–1108
Pace CN, Fu H, Lee Fryar K, Landua J, Trevino SR, Schell D, Thurlkill RL et al (2014) Contribution of hydrogen bonds to protein stability. Protein Sci 23(5):652–661
Maisuradze GG, Liwo A, Scheraga HA (2009) Principal component analysis for protein folding dynamics. J Mol Biol 385(1):312–329
Qais FA, Ahmad I (2019) Mechanism of non-enzymatic antiglycation action by coumarin: a biophysical study. New J Chem 43(32):12823–12835
Qais FA, Alam MM, Naseem I, Ahmad I (2016) Understanding the mechanism of non-enzymatic glycation inhibition by cinnamic acid: an in vitro interaction and molecular modelling study. RSC Adv 6(70):65322–65337
Ahmed A, Shamsi A, Khan MS, Husain FM, Bano B (2019) Probing the interaction of human serum albumin with iprodione, a fungicide: spectroscopic and molecular docking insight. J Biomol Struct Dyn 37(4):857–862
Amani S, Shamsi A, Rabbani G, Naim A (2014) An insight into the biophysical characterization of insoluble collagen aggregates: implication for arthritis. J Fluoresc 24(5):1423–1431
Almutairi FM, Ajmal MR, Siddiqi MK, Amir M, Khan RH (2020) Multi-spectroscopic and molecular docking technique study of the azelastine interaction with human serum albumin. J Mol Struct 1201:127147
Rehman MT, Shamsi H, Khan AU (2014) Insight into the binding mechanism of imipenem to human serum albumin by spectroscopic and computational approaches. Mol Pharm 11(6):1785–1797
Sarzehi S, Chamani J (2010) Investigation on the interaction between tamoxifen and human holo-transferrin: determination of the binding mechanism by fluorescence quenching, resonance light scattering and circular dichroism methods. Int J Biol Macromol 47(4):558–569
Anwar S, Mohammad T, Shamsi A, Queen A, Parveen S, Luqman S, Hasan GM, Alamry KA et al (2020) Discovery of hordenine as a potential inhibitor of pyruvate dehydrogenase kinase 3: implication in lung cancer therapy. Biomedicines 8(5):119
Anwar S, Shamsi A, Shahbaaz M, Queen A, Khan P, Hasan GM, Islam A, Alajmi MF et al (2020) Rosmarinic acid exhibits anticancer effects via MARK4 inhibition. Sci Rep 10(1):1–13
Shamsi A, Furkan M, Khan RH, Khan MS, Shahwan M, Yadav DK (2023) Comprehensive insight into the molecular interaction of rutin with human transferrin: implication of natural compounds in neurodegenerative diseases. Int J Biol Macromol 253:126643
Acknowledgements
MSK acknowledges and extends their appreciation to the Researchers Supporting Project Number (RSP2023R352), King Saud University, Riyadh, Saudi Arabia, for funding this study. A.S. is grateful to Ajman University, UAE, for supporting this publication.
Funding
MSK acknowledges and extends their appreciation to the Researchers Supporting Project Number (RSP2023R352), King Saud University, Riyadh, Saudi Arabia, for funding this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Anas Shamsi, Mohd Shahnawaz Khan, Debarati DasGupta, and Moyad Shahwan. The first draft of the manuscript was written by Anas Shamsi, KM Abdullah, and Debarati DasGupta. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shamsi, A., Shahwan, M., Das Gupta, D. et al. Implication of Caffeic Acid for the Prevention and Treatment of Alzheimer’s Disease: Understanding the Binding with Human Transferrin Using In Silico and In Vitro Approaches. Mol Neurobiol 61, 2176–2185 (2024). https://doi.org/10.1007/s12035-023-03696-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03696-y