Abstract
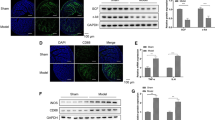
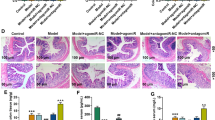
Enteric glial cells (EGCs) are the major component of the enteric nervous system and affect the pathophysiological process of intestinal motility dysfunction. MicroRNAs (miRNAs) play an important role in regulating gastrointestinal homeostasis. However, the mechanism of miRNA-mediated regulation of EGCs in intestinal dysmotility remains unclear. In this study, we investigated the effect of EGC apoptosis on intestinal dysmotility, and the effect of miR-26b-3p on EGC proliferation and apoptosis in vivo and in vitro. A loperamide hydrochloride (Lop)–induced constipated mouse model and an in vitro culture system of rat EGCs were established. The transcriptome was used to predict the differentially expressed gene miR-26b-3p and the target gene Frizzled 10 (FZD10), and their targeting binding relationship was verified by luciferase. EGCs were transfected with miR-26b-3p mimic or antagomir, and the FZD10 expression was down-regulated by siRNA. Immunofluorescence and flow cytometry were used to detect EGC apoptosis. MiR-26b-3p and FZD10 expressions were examined using quantitative real-time PCR (qRT-PCR). The CCK-8 assay was used to detect EGC proliferation. The protein levels were detected by Western blotting and enzyme-linked immunosorbent assay (ELISA). The results showed that miR-26b-3p was up-regulated in the Lop group, whereas FZD10 was down-regulated, and EGC apoptosis was increased in the colon of intestinal dysmotility mice. FZD10 down-regulation and miR-26b-3p mimic significantly increased glycogen synthase kinase-3β phosphorylation (p-GSK3β) levels, decreased β-catenin expression, and promoted EGC apoptosis. MiR-26b-3p antagomir alleviated intestinal dysmotility, promoted EGC increased activity of EGCs, and reduced EGC apoptosis in vivo. In conclusion, this study indicated that miR-26b-3p promotes intestinal motility disorders by targeting FZD10 to block GSK3β/β-catenin signaling and induces apoptosis in EGCs. Our results provide a new research target for the treatment and intervention of intestinal dysmotility.








Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BDNF:
-
Brain-derived neurotrophic factor
- DEMs:
-
Differentially expressed miRNAs
- EGCs:
-
Enteric glial cells
- ELISA:
-
Enzyme-linked immunosorbent assay
- FZD10:
-
Frizzled 10
- GSK3:
-
Glycogen synthase kinase 3
- GDNF:
-
Glial cell–derived neurotrophic factor
- GSNO:
-
S-Nitrosoglutathione
- GFAP:
-
Glial fibrillary acidic protein
- Lop:
-
Loperamide hydrochloride
- miRNAs:
-
MicroRNAs
- NGF:
-
Nerve growth factor
- p-GSK3β:
-
Glycogen synthase kinase-3β phosphorylation
- qRT-PCR:
-
Quantitative real-time PCR
- S100B:
-
S100B protein
References
Schneider S, Wright CM, Heuckeroth RO (2019) Unexpected roles for the second brain: enteric nervous system as master regulator of bowel function. Annu Rev Physiol 81:235–259. https://doi.org/10.1146/annurev-physiol-021317-121515
Liu C, Yang J (2022) Enteric glial cells in immunological disorders of the gut. Front Cell Neurosci 16:895871. https://doi.org/10.3389/fncel.2022.895871
MacEachern SJ, Patel BA, Keenan CM, Dicay M, Chapman K, McCafferty DM, Savidge TC, Beck PL et al (2015) Inhibiting inducible nitric oxide synthase in enteric glia restores electrogenic ion transport in mice with colitis. Gastroenterology 149(2):445-455.e443. https://doi.org/10.1053/j.gastro.2015.04.007
Hu S, Zhao ZK, Liu R, Wang HB, Gu CY, Luo HM, Wang H, Du MH et al (2015) Electroacupuncture activates enteric glial cells and protects the gut barrier in hemorrhaged rats. World J Gastroenterol 21(5):1468–1478. https://doi.org/10.3748/wjg.v21.i5.1468
Rao M, Rastelli D, Dong L, Chiu S, Setlik W, Gershon MD, Corfas G (2017) Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology 153(4):1068-1081.e1067. https://doi.org/10.1053/j.gastro.2017.07.002
Kermarrec L, Durand T, Neunlist M, Naveilhan P, Neveu I (2016) Enteric glial cells have specific immunosuppressive properties. J Neuroimmunol 295–296:79–83. https://doi.org/10.1016/j.jneuroim.2016.04.011
Wang XM, Lv LX, Qin YS, Zhang YZ, Yang N, Wu S, Xia XW, Yang H et al (2022) Ji-Chuan decoction ameliorates slow transit constipation via regulation of intestinal glial cell apoptosis. World J Gastroenterol 28(34):5007–5022. https://doi.org/10.3748/wjg.v28.i34.5007
Bassotti G, Villanacci V, Nascimbeni R, Cadei M, Fisogni S, Antonelli E, Corazzi N, Salerni B (2009) Enteric neuroglial apoptosis in inflammatory bowel diseases. J Crohns Colitis 3(4):264–270. https://doi.org/10.1016/j.crohns.2009.06.004
Kim YT, Hur EM, Snider WD, Zhou FQ (2011) Role of GSK3 signaling in neuronal morphogenesis. Front Mol Neurosci 4:48. https://doi.org/10.3389/fnmol.2011.00048
Hur EM, Zhou FQ (2010) GSK3 signalling in neural development. Nat Rev Neurosci 11(8):539–551. https://doi.org/10.1038/nrn2870
Sutherland C (2011) What are the bona fide GSK3 substrates? Int J Alzheimers Dis 2011:505607. https://doi.org/10.4061/2011/505607
Dorfman T, Pollak Y, Sohotnik R, Coran AG, Bejar J, Sukhotnik I (2015) Enhanced intestinal epithelial cell proliferation in diabetic rats correlates with β-catenin accumulation. J Endocrinol 226(3):135–143. https://doi.org/10.1530/JOE-14-0725
Li B, Lee C, Cadete M, Zhu H, Koike Y, Hock A, Wu RY, Botts SR et al (2019) Impaired Wnt/β-catenin pathway leads to dysfunction of intestinal regeneration during necrotizing enterocolitis. Cell Death Dis 10(10):743. https://doi.org/10.1038/s41419-019-1987-1
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297. https://doi.org/10.1016/s0092-8674(04)00045-5
Su J, Zhang A, Shi Z, Ma F, Pu P, Wang T, Zhang J, Kang C et al (2012) MicroRNA-200a suppresses the Wnt/β-catenin signaling pathway by interacting with β-catenin. Int J Oncol 40(4):1162–1170. https://doi.org/10.3892/ijo.2011.1322
Zhou Q, Verne GN (2011) miRNA-based therapies for the irritable bowel syndrome. Expert Opin Biol Ther 11(8):991–995. https://doi.org/10.1517/14712598.2011.577060
Xiao X, Mao X, Chen D, Yu B, He J, Yan H, Wang J (2022) miRNAs can affect intestinal epithelial barrier in inflammatory bowel disease. Front Immunol 13:868229. https://doi.org/10.3389/fimmu.2022.868229
Zhi X, Tao J, Li Z, Jiang B, Feng J, Yang L, Xu H, Xu Z (2014) MiR-874 promotes intestinal barrier dysfunction through targeting AQP3 following intestinal ischemic injury. FEBS Lett 588(5):757–763. https://doi.org/10.1016/j.febslet.2014.01.022
Chao G, Wang Y, Zhang S, Yang W, Ni Z, Zheng X (2017) MicroRNA-29α increased the intestinal membrane permeability of colonic epithelial cells in irritable bowel syndrome rats. Oncotarget 8(49):85828–85837. https://doi.org/10.18632/oncotarget.20687
Geng F, Lu GF, Ji MH, Kong DY, Wang SY, Tian H, Xie ZM, Pan M et al (2019) MicroRNA-26b-3p/ANTXR1 signaling modulates proliferation, migration, and apoptosis of glioma. Am J Transl Res 11(12):7568–7578
Koike J, Takagi A, Miwa T, Hirai M, Terada M, Katoh M (1999) Molecular cloning of Frizzled-10, a novel member of the Frizzled gene family. Biochem Biophys Res Commun 262(1):39–43. https://doi.org/10.1006/bbrc.1999.1161
Xavier CP, Melikova M, Chuman Y, Uren A, Baljinnyam B, Rubin JS (2014) Secreted Frizzled-related protein potentiation versus inhibition of Wnt3α/β-catenin signaling. Cell Signal 26(1):94–101. https://doi.org/10.1016/j.cellsig.2013.09.016
Zhao Q, Xing F, Tao Y, Liu H, Huang K, Peng Y, Feng N, Liu C (2020) Xiaozhang Tie improves intestinal motility in rats with cirrhotic ascites by regulating the stem cell factor/c-kit pathway in interstitial cells of Cajal. Front Pharmacol 11:1. https://doi.org/10.3389/fphar.2020.00001
Chen JD, Yin J, Wei W (2017) Electrical therapies for gastrointestinal motility disorders. Expert Rev Gastroenterol Hepatol 11(5):407–418. https://doi.org/10.1080/17474124.2017.1298441
Ochoa-Cortes F, Turco F, Linan-Rico A, Soghomonyan S, Whitaker E, Wehner S, Cuomo R, Christofi FL (2016) Enteric glial cells: a new frontier in neurogastroenterology and clinical target for inflammatory bowel diseases. Inflamm Bowel Dis 22(2):433–449. https://doi.org/10.1097/MIB.0000000000000667
Zhan Y, Wen Y, Du LJ, Wang XX, Tang SY, Kong PF, Huang WG, Tang XG (2022) Effects of Maren pills on the intestinal microflora and short-chain fatty acid profile in drug-induced slow transit constipation model rats. Front Pharmacol 13:804723. https://doi.org/10.3389/fphar.2022.804723
Bassotti G, Villanacci V (2011) Can “functional” constipation be considered as a form of enteric neuro-gliopathy? Glia 59(3):345–350. https://doi.org/10.1002/glia.21115
Brenner M, Messing A (2021) Regulation of GFAP expression. ASN Neuro 13:1–32. https://doi.org/10.1177/1759091420981206
Robinson AM, Stojanovska V, Rahman AA, McQuade RM, Senior PV, Nurgali K (2016) Effects of oxaliplatin treatment on the enteric glial cells and neurons in the mouse ileum. J Histochem Cytochem 64(9):530–545. https://doi.org/10.1369/0022155416656842
Budni J, Bellettini-Santos T, Mina F, Garcez ML, Zugno AI (2015) The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis 6(5):331–341. https://doi.org/10.14336/AD.2015.0825
Yao L, Lu P, Ling EA (2016) Melatonin suppresses toll like receptor 4-dependent caspase-3 signaling activation coupled with reduced production of proinflammatory mediators in hypoxic microglia. PLoS One 11(11):e0166010. https://doi.org/10.1371/journal.pone.0166010
Zheng TS, Schlosser SF, Dao T, Hingorani R, Crispe IN, Boyer JL, Flavell RA (1998) Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proc Natl Acad Sci U S A 95(23):13618–13623. https://doi.org/10.1073/pnas.95.23.13618
Alrefaei AF, Munsterberg AE, Wheeler GN (2020) FZD10 regulates cell proliferation and mediates Wnt1 induced neurogenesis in the developing spinal cord. PLoS One 15(6):e0219721. https://doi.org/10.1371/journal.pone.0219721
Yan Y, Li Y, Hu C, Gu X, Liu J, Hu YA, Yang Y, Wei Y et al (2009) Expression of Frizzled10 in mouse central nervous system. Gene Expr Patterns 9(3):173–177. https://doi.org/10.1016/j.gep.2008.11.001
Hu H, Zhao P, Liu J, Ke Q, Zhang C, Guo Y, Ding H (2018) Lanthanum phosphate/chitosan scaffolds enhance cytocompatibility and osteogenic efficiency via the Wnt/β-catenin pathway. J Nanobiotechnol 16(1):98. https://doi.org/10.1186/s12951-018-0411-9
Funding
This work was supported by the Youth Program of National Natural Science Foundation of China (No. 82004173), the National Natural Science Foundation of China (No. 82074429), the Science and technology Research Special project of Sichuan provincial Administration of Traditional Chinese Medicine (No. 2020JC0063), and the Science and Technology Project of Luzhou City (2022-SYF-99).
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly to this work. YZ and YW contributed equally to this work. YZ, YW, and XGT conceived and designed the experiments; FZ, LJD, and TYC performed most of the experiments; LJD and TYC contributed to data collection; XLS and RW analyzed the data; YZ and YW wrote the manuscript; XGT revised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The procedures in this experiment were performed in accordance with protocols approved by the Animal Ethics Committee of West China Hospital of Sichuan University (Approval No. 20221203006).
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yu Zhan and Yong Wen contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhan, Y., Wen, Y., Zheng, F. et al. MiR-26b-3p Promotes Intestinal Motility Disorder by Targeting FZD10 to Inhibit GSK3β/β-Catenin Signaling and Induce Enteric Glial Cell Apoptosis. Mol Neurobiol 61, 1543–1561 (2024). https://doi.org/10.1007/s12035-023-03600-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03600-8