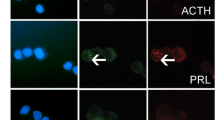
Abstract
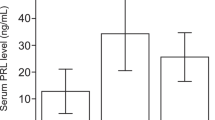
Aromatase enzyme plays an essential role in estrogen-induced tumorigenesis. It is expressed in the normal pituitary and more significantly in prolactinoma tissues. The aim of this study was to investigate the effects of an aromatase inhibitor, letrozole, on MMQ and GH3 rat prolactinoma cell lines and evaluate the possible mechanism of action. MMQ and GH3 cells were characterized with demonstrating aromatase enzyme and estrogen receptor alpha expression by PCR and immunofluorescence staining. After dose optimization for testosterone (T) and letrozole (L), four groups were established: only the testosteron-treated group (T) to detect cell proliferation; only letrozole-treated group (L) to investigate apoptotic effects; testosterone and letrozole concomitant-treated group to demonstrate inhibition of testosterone induced cell proliferation with letrozole treatment s(T + L) and control group (C) with no treatment. The proliferation rate of cells was determined by WST-1. For the detection of apoptotic and necrotic cells, Annexin V and caspase-3 labeling was used. Prolactin and estrogen levels were measured with ELISA, and the mRNA expression of aromatase and Esr1 was also determined. Testosterone induced the proliferation of MMQ and GH3 cells and further increased prolactin and estradiol levels. Adding letrozole to testosterone resulted in decreased cellular proliferation and even induced apoptosis. Also, letrozole administration significantly decreased prolactin and estradiol levels. However, letrozole alone had no effects on proliferation and apoptosis. Gene expression of aromatase and Esr1 was also significantly decreased by letrozole treatment. This in vitro study demonstrated that treatment of testosterone proliferating cells with letrozole resulted in decreased prolactin levels and cell proliferation and induced apoptosis, and further loss of aromatase and Esr1 mRNA expression were observed. Although this is an in vivo study, the results showed unique and novel findings which may easily be adapted to clinical use for further verification.











Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Freeman ME, Kanyicska L, Lerant A, Gy A, Nagy R, Kanyicska B et al (2000) Prolactin: structure, function, and regulation of secretion. Physiol Rev 80(4):1523–1631
Levin G (2018) Prolactin, prolactin disorders, and dopaminee agonists during pregnancy. Horm 18(2):137–139
Matsushita N, Kato Y, Shimatsu A, Katakami H, Yanaihara N, Imura H (1983) Effects of VIP, TRH, GABA and dopaminee on prolactin release from superfused rat anterior pituitary cells. Life Sci 32(11):1263–1269
Vale W, Blackwell R, Grant G, Guillemin R (1973) TRF and thyroid hormones on prolactin secretion by rat anterior pituitary cells in vitro. Endocrinology 93(1):26–33
Vilar L, Abucham J, Albuquerque JL, Araujo LA, Azevedo MF, Boguszewski CL et al (2018) Controversial issues in the management of hyperprolactinemia and prolactinomas – an overview by the Neuroendocrinology Department of the Brazilian Society of Endocrinology and Metabolism. Arch Endocrinol Metab 62(2):236–263
Glezer A, Bronstein MD (2015) Prolactinomas. Endocrinol Metab Clin North Am 44(1):71–78
Guo Q, Erickson BJ, Chang ÞAY, Erickson D (2015) Characteristics of dynamic magnetic resonance ımage enhancement in prolactinomas resistant to dopaminee agonist therapy. J Investig Med 63(3):529–534
Gürlek A, Karavitaki N, Ansorge O, Wass JAH (2007) What are the markers of aggressiveness in prolactinomas? Changes in cell biology, extracellular matrix components, angiogenesis and genetics. Eur J Endocrinol 156(2):143–53
Sodi R, Fikri R, Diver M, Ranganath L, Vora J (2005) Case report testosterone replacement-induced hyperprolactinaemia : case report and review of the literature. Ann Clin Biochem 42:153–159
Goh HH, Ratnam SS (1990) Effect of estrogens on prolactin secretion in transsexual subjects. Arch Sex Behav 19(5):507–516
Sánchez M, Suárez L, Cantabrana B, Bordallo J (2017) Estradiol-modified prolactin secretion independently of action potentials and Ca2 + and blockade of outward potassium currents in GH3cells. Naunyn Schmiedebergs Arch Pharmacol 390(1):95–104
Mueller A, Gooren L (2008) Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol 159(3):197–202
Molitch ME (2014) Management of medically refractory prolactinoma. J Neurooncol 117(3):421–428
Asscheman H, Gooren LJ, Assies J, Smits JP, de Slegte R (1988) Prolactin levels and pituitary enlargement in hormone-treated male-to-female transsexuals. Clin Endocrinol (Oxf) 28(6):583–588
Chao W, Zhi-qiang H, Ming C, Zhi W, Wei-guang Z, Lai-zang W et al (2012) Resveratrol inhibited GH3 cell growth and decreased prolactin level via estrogen receptors. Clin Neurol Neurosurg 114(3):241–248
Wang C, Xu J, Wen Y, Zhang D, Wang X, Chang L et al (2018) Fulvestrant inhibits the glycolysis of prolactinoma GH3 cells by downregulating IRE1 / XBP1 signaling pathway. Eur Rev Med Pharmacol Sci 22:5364–5370
Leng L, Zhang Y (2011) Effects of an estrogen receptor antagonist on proliferation, prolactin secretion and growth factor expression in the MMQ pituitary prolactinoma cell line. J Clin Neurosci 18(12):1694–1698
Thiantanawat A, Long BJ, Brodie AM (2003) Signaling pathways of apoptosis activated by aromatase ınhibitors and antiestrogens. Cancer Res 63(22):8037–8050
Amaral C, Borges M, Melo S, da Silva ET, Correia-da-Silva G, Teixeira N (2012) Apoptosis and autophagy in breast cancer cells following exemestane treatment. PLoS One 7(8):1–10
Santen RJ, Yue W, Wang JP (2015) Estrogen metabolites and breast cancer. Steroids 99:61–6
Carretero J, Burks DJ, Vázquez G, Rubio M, Hernández E, Bodego P et al (2002) Expression of aromatase P450 is increased in spontaneous prolactinomas of aged rats. Pituitary 5(1):5–10
Sasano H, Takahashi K, Satoh F, Nagura H, Harada N (1988) Aromatase in the human central nervous system. Clin Endocrinol (Oxf) 48(3):325–329
Kadioglu P, Oral G, Sayitoglu M, Erensoy N, Senel B, Gazioglu N et al (2008) Aromatase cytochrome P450 enzyme expression in human pituitary. Pituitary 11(1):29–35
Akinci H, Kapucu A, Dar KA, Celik O, Tutunculer B, Sirin G et al (2013) Aromatase cytochrome P450 enzyme expression in prolactinomas and its relationship to tumor behavior. Pituitary 16(3):386–392
Selek A, Cetinarslan B, Gurbuz Y, Tarkun I, Canturk Z, Cabuk B (2015) Aromatase enzyme expression in acromegaly and its possible relationship with disease prognosis. Endocrine 49(1):250–251
Strange F, Remonda L, Schu P, Fandino J, Berkmann S (2019) 10 years’ experience of using low-field intraoperative MRI in transsphenoidal surgery for pituitary adenoma: results of the Swiss Pituitary Registry (SwissPit). World Neurosurg 136:284–293
Wang X, Du Q, Mao Z, Fan X, Hu B, Wang Z et al (2017) Combined treatment with artesunate and bromocriptine has synergistic anticancer effects in pituitary adenoma cell lines. Oncotarget 8(28):45874–45887
Zhang D, Way JS, Zhang X, Sergey M, Bergsneider M, Wang MB et al (2019) Effect of everolimus in treatment of aggressive prolactin-secreting pituitary adenomas. J Clin Endocrinol Metab 104(January):1929–1936
Coopmans EC, Van Meyel SWF, Pieterman KJ, Van Ipenburg JA, Hofland LJ, Donga E et al (2019) Excellent response to pasireotide therapy in an aggressive and dopaminee-resistant prolactinoma. Eur J Endocrinol 181(2):21–27
Gillam MP, Middler S, Freed DJ, Molitch ME (2002) The novel use of very high doses of cabergoline and a combination of testosterone and an aromatase ınhibitor in the treatment of a giant prolactinoma. J Clin Endocrinol Metab 87(10):4447–4451
Heidari Z, Hosseinpanah F, Shirazian N (2010) Achievement of fertility in an infertile man with resistant macroprolactinoma using high-dose bromocriptine and a combination of Human Chorıonıc Gonadotropın and an aromatase inhibitor. Endocr Pract 16(4):669–672
Su Y, Du G, Shen H, Wang W, Bao J, Aierken A et al (2019) Increased expression of aromatase cytochrome P450 enzyme is associated with prolactinoma invasiveness in post-menopausal women. J Int Med Res 47(7):3115–3126
García-Barrado MJ, Blanco EJ, Iglesias-Osma MC, Carretero-Hernández M, Catalano-Iniesta L, Sanchez-Robledo V et al (2017) Relation among aromatase P450 and tumoral growth in human prolactinomas. Int J Mol Sci 18(11):1–16
García Barrado MJ, Blanco EJ, Carretero Hernández M, Iglesias Osma MC, Carretero M, Herrero JJ et al (2014) Local transformations of androgens into estradiol by aromatase P450 is involved in the regulation of prolactin and the proliferation of pituitary prolactin-positive cells. PLoS ONE 9(6):1–9
Zhang X, Xu W, Su J, Chu M, Jin H, Li G et al (2014) The prosurvival role of autophagy in resveratrol-induced cytotoxicity in GH3 cells. Int J Mol Med 33(4):987–993
Rodríguez-Lozano DC, Piña-Medina AG, Hansberg-Pastor V, Bello-Alvarez C, Camacho-Arroyo I (2019) Testosterone Promotes glioblastoma cell proliferation, migration, and ınvasion through androgen receptor activation. Front Endocrinol (Lausanne) 4(10):16. https://doi.org/10.3389/fendo.2019.00016
Acknowledgements
Special thanks is given to Novo-Nordisk for collaboration.
Funding
The study was supported by the Research Fund of the University and Novo-Nordisk collaboration, project no. 2013/155.
Author information
Authors and Affiliations
Contributions
Alev Selek had the idea for the article and drafted it, performed the literature search, data analysis, and writing process, and also revised the work. Zehra Seda Unal Halbutoğulları had literature search, data analysis, and writing process. Çiğdem İnci Aydemir had literature search, main cell culture, apopitosis studies, and writing process. Berrin Cetinarslan had literature search, data interpretation, and discussion. Zeynep antürk had literature search, data interpretation, and discussion. İlhan Tarkun had literature search, data interpretation, and discussion. Gülay Erman, had literature search, ELISA studies, and writing process. Cansu Subaşı had literature search, PCR studies, and writing process. Erdal Karaöz had literature search, data interpretation, and discussion and also revision.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors. The study protocol was approved by the local ethics committee.
Consent to Participate
All authors have consent to participate in the study.
Consent for Publication
All authors have consent for publication of the study.
Competing Interests
All authors have declared that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Selek, A., Halbutoğulları, Z.S.U., Aydemir, Ç.İ. et al. Letrozole Decreased Testosterone-Induced Cell Proliferation and Prolactin Secretion also Increased Apoptosis in MMQ and GH3 Rat Prolactinoma Cell Lines. Mol Neurobiol 60, 2442–2454 (2023). https://doi.org/10.1007/s12035-023-03220-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03220-2