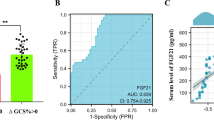
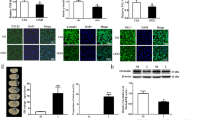
Abstract
Hyperglycemia is a risk factor for poor prognosis after acute ischemic stroke and promote the occurrence of hemorrhagic transformation (HT). The activation of P2RX7 play an important role in endotheliocyte damage and BBB disruption. Ferroptosis is a novel pattern of programmed cell death caused by the accumulation of intracellular iron and lipid peroxidation, resulting in ROS production and cell death. This study is to explore the mechanism of P2RX7 in reducing HT pathogenesis after acute ischemic stroke through regulating endotheliocyte ferroptosis. Male SD rats were performed to establish middle cerebral artery occlusion (MCAO) model injected with 50% high glucose (HG) and HUVECs were subjected to OGD/R treated with high glucose (30 mM) for establishing HT model in vivo and in vitro. P2RX7 inhibitor (BBG), and P2RX7 small interfering RNAs (siRNA) were used to investigate the role of P2RX7 in BBB after MCAO in vivo and OGD/R in vitro, respectively. The neurological deficits, infarct volume, degree of intracranial hemorrhage, integrity of the BBB, immunoblotting, and immunofluorescence were evaluated at 24 h after MCAO. Our study found that the level of P2RX7 was gradually increased after MCAO and/or treated with HG. Our results showed that treatment with HG after MCAO can aggravate neurological deficits, infarct volume, oxidative stress, iron accumulation, and BBB injury in HT model, and HG-induced HUVECs damage. The inhibition of P2RX7 reversed the damage effect of HG, significantly downregulated the expression level of P53, HO-1, and p-ERK1/2 and upregulated the level of SLC7A11 and GPX4, which implicated that P2RX7 inhibition could attenuate oxidative stress and ferroptosis of endothelium in vivo and in vitro. Our data provided evidence that the P2RX7 play an important role in HG-associated oxidative stress, endothelial damage, and BBB disruption, which regulates HG-induced HT by ERK1/2 and P53 signaling pathways after MCAO.










Similar content being viewed by others
Data Availability
The datasets supporting the conclusions of this article are included within the article and its additional files. All material used in this manuscript will be made available to researchers subject to confidentiality.
Change history
23 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12035-022-03138-1
References
Couret D, Bourane S, Catan A, Nativel B, Planesse C, Dorsemans AC, Ait-Arsa I, Cournot M et al (2018) A hemorrhagic transformation model of mechanical stroke therapy with acute hyperglycemia in mice. J Comp Neurol 526(6):1006–1016. https://doi.org/10.1002/cne.24386
Shao A, Gao S, Wu H, Xu W, Pan Y, Fang Y, Wang X, Zhang J (2021) Melatonin Ameliorates hemorrhagic transformation via suppression of ROS-induced NLRP3 activation after cerebral ischemia in hyperglycemic rats. Oxid Med Cell Longev 2021:6659282. https://doi.org/10.1155/2021/6659282
Weiland A, Wang Y, Wu W, Lan X, Han X, Li Q, Wang J (2019) Ferroptosis and its role in diverse brain diseases. Mol Neurobiol 56(7):4880–4893. https://doi.org/10.1007/s12035-018-1403-3
Su L, Jiang X, Yang C, Zhang J, Chen B, Li Y, Yao S, Xie Q et al (2019) Pannexin 1 mediates ferroptosis that contributes to renal ischemia/reperfusion injury. J Biol Chem 294(50):19395–19404. https://doi.org/10.1074/jbc.RA119.010949
Cheng Y, Song Y, Chen H, Li Q, Gao Y, Lu G, Luo C (2021) Ferroptosis mediated by lipid reactive oxygen species: a possible causal link of neuroinflammation to neurological disorders. Oxid Med Cell Longev 2021:5005136. https://doi.org/10.1155/2021/5005136
Chen B, Chen Z, Liu M, Gao X, Cheng Y, Wei Y, Wu Z, Cui D et al (2019) Inhibition of neuronal ferroptosis in the acute phase of intracerebral hemorrhage shows long-term cerebroprotective effects. Brain Res Bull 153:122–132. https://doi.org/10.1016/j.brainresbull.2019.08.013
Feng Y, Madungwe NB, Imam Aliagan AD, Tombo N, Bopassa JC (2019) Liproxstatin-1 protects the mouse myocardium against ischemia/reperfusion injury by decreasing VDAC1 levels and restoring GPX4 levels. Biochem Biophys Res Commun 520(3):606–611. https://doi.org/10.1016/j.bbrc.2019.10.006
Ma H, Wang X, Zhang W, Li H, Zhao W, Sun J, Yang M (2020) Melatonin suppresses ferroptosis induced by high glucose via activation of the Nrf2/HO-1 signaling pathway in type 2 diabetic osteoporosis. Oxid Med Cell Longev 2020:9067610. https://doi.org/10.1155/2020/9067610
He J, Li Z, Xia P, Shi A, FuChen X, Zhang J, Yu P (2022) Ferroptosis and ferritinophagy in diabetes complications. Mol Metab 60:101470. https://doi.org/10.1016/j.molmet.2022.101470
Sperlágh B, Illes P (2014) P2X7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol Sci 35(10):537–547. https://doi.org/10.1016/j.tips.2014.08.002
Zhao H, Zhang X, Dai Z, Feng Y, Li Q, Zhang JH, Liu X, Chen Y et al (2016) P2X7 receptor suppression preserves blood-brain barrier through inhibiting RhoA activation after experimental intracerebral hemorrhage in rats. Sci Rep 6:23286. https://doi.org/10.1038/srep23286
Huang C, Chi XS, Li R, Hu X, Xu HX, Li JM, Zhou D (2017) Inhibition of P2X7 receptor ameliorates nuclear factor-kappa B mediated neuroinflammation induced by status epilepticus in rat hippocampus. J Mol Neurosci 63(2):173–184. https://doi.org/10.1007/s12031-017-0968-z
Wen Z, Mei B, Li H, Dou Y, Tian X, Shen M, Chen G (2017) P2X7 Participates in Intracerebral hemorrhage-induced secondary brain injury in rats via MAPKs signaling pathways. Neurochem Res 42(8):2372–2383. https://doi.org/10.1007/s11064-017-2257-1
Furuta T, Mukai A, Ohishi A, Nishida K, Nagasawa K (2017) Oxidative stress-induced increase of intracellular zinc in astrocytes decreases their functional expression of P2X7 receptors and engulfing activity. Metallomics 9(12):1839–1851. https://doi.org/10.1039/c7mt00257b
Leng B, Li C, Sun Y, Zhao K, Zhang L, Lu ML, Wang HX (2020) Protective effect of astragaloside IV on high glucose-induced endothelial dysfunction via inhibition of P2X7R dependent P38 MAPK signaling pathway. Oxid Med Cell Longev 2020:5070415. https://doi.org/10.1155/2020/5070415
Deng H, Zhang Y, Li GG, Yu HH, Bai S, Guo GY, Guo WL, Ma Y et al (2021) P2X7 receptor activation aggravates NADPH oxidase 2-induced oxidative stress after intracerebral hemorrhage. Neural Regen Res 16(8):1582–1591. https://doi.org/10.4103/1673-5374.303036
Rawish E, Langer HF (2022) Platelets and the role of P2X receptors in nociception, pain, neuronal toxicity and thromboinflammation. Int J Mol Sci 23(12). https://doi.org/10.3390/ijms23126585
Hirayama Y, Anzai N, Koizumi S (2021) Mechanisms underlying sensitization of P2X7 receptors in astrocytes for induction of ischemic tolerance. Glia 69(9):2100–2110. https://doi.org/10.1002/glia.23998
Mekala N, Gheewala N, Rom S, Sriram U, Persidsky Y (2022) Blocking of P2X7r reduces mitochondrial stress induced by alcohol and electronic cigarette exposure in brain microvascular endothelial cells. Antioxidants (Basel) 11(7). https://doi.org/10.3390/antiox11071328
Wang W, Li M, Wang Y, Li Q, Deng G, Wan J, Yang Q, Chen Q et al (2016) GSK-3β inhibitor TWS119 attenuates rtPA-induced hemorrhagic transformation and activates the Wnt/β-catenin signaling pathway after acute ischemic stroke in rats. Mol Neurobiol 53(10):7028–7036. https://doi.org/10.1007/s12035-015-9607-2
Liu C, Sun S, Xie J, Li H, Li T, Wu Q, Zhang Y, Bai X et al (2022) GLP-1R Agonist exendin-4 protects against hemorrhagic transformation induced by rtPA after ischemic stroke via the Wnt/β-catenin signaling pathway. Mol Neurobiol. https://doi.org/10.1007/s12035-022-02811-9
Yasmin A, Pitkänen A, Andrade P, Paananen T, Gröhn O, Immonen R (2021) Post-injury ventricular enlargement associates with iron in choroid plexus but not with seizure susceptibility nor lesion atrophy-6-month MRI follow-up after experimental traumatic brain injury. Brain Struct Funct. https://doi.org/10.1007/s00429-021-02395-5
Prasad S, Sajja RK, Park JH, Naik P, Kaisar MA, Cucullo L (2015) Impact of cigarette smoke extract and hyperglycemic conditions on blood-brain barrier endothelial cells. Fluids Barriers CNS 12:18. https://doi.org/10.1186/s12987-015-0014-x
Malik S, Saha R, Seth P (2014) Involvement of extracellular signal-regulated kinase (ERK1/2)-p53-p21 axis in mediating neural stem/progenitor cell cycle arrest in co-morbid HIV-drug abuse exposure. J Neuroimmune Pharmacol 9(3):340–353. https://doi.org/10.1007/s11481-014-9523-7
Park E, Chung SW (2019) ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis 10(11):822. https://doi.org/10.1038/s41419-019-2064-5
Saralkar P, Arsiwala T, Geldenhuys WJ (2020) Nanoparticle formulation and in vitro efficacy testing of the mitoNEET ligand NL-1 for drug delivery in a brain endothelial model of ischemic reperfusion-injury. Int J Pharm 578:119090. https://doi.org/10.1016/j.ijpharm.2020.119090
Tang YC, Tian HX, Yi T, Chen HB (2016) The critical roles of mitophagy in cerebral ischemia. Protein Cell 7(10):699–713. https://doi.org/10.1007/s13238-016-0307-0
Ding H, Yan CZ, Shi H, Zhao YS, Chang SY, Yu P, Wu WS, Zhao CY et al (2011) Hepcidin is involved in iron regulation in the ischemic brain. PLoS ONE 6(9):e25324. https://doi.org/10.1371/journal.pone.0025324
Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL, Liuyang ZY, Roisman L et al (2017) Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry 22(11):1520–1530. https://doi.org/10.1038/mp.2017.171
Chen W, Jiang L, Hu Y, Tang N, Liang N, Li XF, Chen YW, Qin H et al (2021) Ferritin reduction is essential for cerebral ischemia-induced hippocampal neuronal death through p53/SLC7A11-mediated ferroptosis. Brain Res 1752:147216. https://doi.org/10.1016/j.brainres.2020.147216
Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, Cheng T, Yang J et al (2017) Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2(7):e90777. https://doi.org/10.1172/jci.insight.90777
Li Q, Weiland A, Chen X, Lan X, Han X, Durham F, Liu X, Wan J et al (2018) Ultrastructural characteristics of neuronal death and white matter injury in mouse brain tissues after intracerebral hemorrhage: coexistence of ferroptosis, autophagy, and necrosis. Front Neurol 9:581. https://doi.org/10.3389/fneur.2018.00581
Abdul Y, Li W, Ward R, Abdelsaid M, Hafez S, Dong G, Jamil S, Wolf V et al (2021) Deferoxamine treatment prevents post-stroke vasoregression and neurovascular unit remodeling leading to improved functional outcomes in type 2 male diabetic rats: role of endothelial ferroptosis. Transl Stroke Res 12(4):615–630. https://doi.org/10.1007/s12975-020-00844-7
Fu C, Wu Y, Liu S, Luo C, Lu Y, Liu M, Wang L, Zhang Y et al (2022) Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J Ethnopharmacol 289:115021. https://doi.org/10.1016/j.jep.2022.115021
Zhai QY, Ren YQ, Ni QS, Song ZH, Ge KL, Guo YL (2022) Transplantation of human umbilical cord mesenchymal stem cells-derived neural stem cells pretreated with neuregulin1β ameliorate cerebral ischemic reperfusion injury in rats. Biomolecules 12(3). https://doi.org/10.3390/biom12030428
Kunte H, Busch MA, Trostdorf K, Vollnberg B, Harms L, Mehta RI, Castellani RJ, Mandava P et al (2012) Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann Neurol 72(5):799–806. https://doi.org/10.1002/ana.23680
Kuang F, Liu J, Tang D, Kang R (2020) Oxidative damage and antioxidant defense in ferroptosis. Front Cell Dev Biol 8:586578. https://doi.org/10.3389/fcell.2020.586578
Luo EF, Li HX, Qin YH, Qiao Y, Yan GL, Yao YY, Li LQ, Hou JT et al (2021) Role of ferroptosis in the process of diabetes-induced endothelial dysfunction. World J Diabetes 12(2):124–137. https://doi.org/10.4239/wjd.v12.i2.124
Zhang J, Qiu Q, Wang H, Chen C, Luo D (2021) TRIM46 contributes to high glucose-induced ferroptosis and cell growth inhibition in human retinal capillary endothelial cells by facilitating GPX4 ubiquitination. Exp Cell Res 407(2):112800. https://doi.org/10.1016/j.yexcr.2021.112800
Chen F, Wang W, Ding H, Yang Q, Dong Q, Cui M (2016) The glucagon-like peptide-1 receptor agonist exendin-4 ameliorates warfarin-associated hemorrhagic transformation after cerebral ischemia. J Neuroinflammation 13(1):204. https://doi.org/10.1186/s12974-016-0661-0
Kuroki T, Tanaka R, Shimada Y, Yamashiro K, Ueno Y, Shimura H, Urabe T, Hattori N (2016) Exendin-4 inhibits matrix metalloproteinase-9 activation and reduces infarct growth after focal cerebral ischemia in hyperglycemic mice. Stroke 47(5):1328–1335. https://doi.org/10.1161/STROKEAHA.116.012934
Arbeloa J, Pérez-Samartín A, Gottlieb M, Matute C (2012) P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis 45(3):954–961. https://doi.org/10.1016/j.nbd.2011.12.014
Seeland S, Kettiger H, Murphy M, Treiber A, Giller J, Kiss A, Sube R, Krähenbühl S et al (2015) ATP-induced cellular stress and mitochondrial toxicity in cells expressing purinergic P2X7 receptor. Pharmacol Res Perspect 3(2):e00123. https://doi.org/10.1002/prp2.123
Ferrazoli EG, de Souza HD, Nascimento IC, Oliveira-Giacomelli Á, Schwindt TT, Britto LR, Ulrich H (2017) Brilliant Blue G, but not fenofibrate, treatment reverts hemiparkinsonian behavior and restores dopamine levels in an animal model of Parkinson’s disease. Cell Transplant 26(4):669–677. https://doi.org/10.3727/096368917X695227
Cieślak M, Wojtczak A (2018) Role of purinergic receptors in the Alzheimer’s disease. Purinergic Signal 14(4):331–344. https://doi.org/10.1007/s11302-018-9629-0
Wu XM, Zhang N, Li JS, Yang ZH, Huang XL, Yang XF (2022) Purinergic receptors mediate endothelial dysfunction and participate in atherosclerosis. Purinergic Signal. https://doi.org/10.1007/s11302-021-09839-x
Sathanoori R, Swärd K, Olde B, Erlinge D (2015) The ATP Receptors P2X7 and P2X4 modulate high glucose and palmitate-induced inflammatory responses in endothelial cells. PLoS ONE 10(5):e0125111. https://doi.org/10.1371/journal.pone.0125111
Zhang QL, Wang W, Alatantuya D, Lu ZJ, Li LL, Zhang TZ (2018) Down-regulated miR-187 promotes oxidative stress-induced retinal cell apoptosis through P2X7 receptor. Int J Biol Macromol 120(Pt A):801–810. https://doi.org/10.1016/j.ijbiomac.2018.08.166
da Silva CS, Calió ML, Mosini AC, Pires JM, Rêgo D, Mello LE, Leslie A (2019) LPS-induced systemic neonatal inflammation: blockage of P2X7R by BBG decreases mortality on rat pups and oxidative stress in hippocampus of adult rats. Front Behav Neurosci 13:240. https://doi.org/10.3389/fnbeh.2019.00240
Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brüne B, Sterzel RB (1998) Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol 275(6):F962-971. https://doi.org/10.1152/ajprenal.1998.275.6.F962
Mello Pde A, Filippi-Chiela EC, Nascimento J, Beckenkamp A, Santana DB, Kipper F, Casali EA, Nejar Bruno A et al (2014) Adenosine uptake is the major effector of extracellular ATP toxicity in human cervical cancer cells. Mol Biol Cell 25(19):2905–2918. https://doi.org/10.1091/mbc.E14-01-0042
Zhang Y, Yuan F, Cao X, Zhai Z, Du GangHuang, X, Wang Y, Zhang J, Huang Y et al (2014) P2X7 receptor blockade protects against cisplatin-induced nephrotoxicity in mice by decreasing the activities of inflammasome components, oxidative stress and caspase-3. Toxicol Appl Pharmacol 281(1):1–10. https://doi.org/10.1016/j.taap.2014.09.016
Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520(7545):57–62. https://doi.org/10.1038/nature14344
Kang R, Kroemer G, Tang D (2019) The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med 133:162–168. https://doi.org/10.1016/j.freeradbiomed.2018.05.074
Hong T, Lei G, Chen X, Li H, Zhang X, Wu N, Zhao Y, Zhang Y et al (2021) PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol 42:101928. https://doi.org/10.1016/j.redox.2021.101928
Kuang H, Wang T, Liu L, Tang C, Li T, Liu M, Wang T, Zhong W et al (2021) Treatment of early brain injury after subarachnoid hemorrhage in the rat model by inhibiting p53-induced ferroptosis. Neurosci Lett 762:136134. https://doi.org/10.1016/j.neulet.2021.136134
Zhu K, Zhu X, Sun S, Yang W, Liu S, Tang Z, Zhang R, Li J et al (2021) Inhibition of TLR4 prevents hippocampal hypoxic-ischemic injury by regulating ferroptosis in neonatal rats. Exp Neurol 345:113828. https://doi.org/10.1016/j.expneurol.2021.113828
Gendron FP, Neary JT, Theiss PM, Sun GY, Gonzalez FA, Weisman GA (2003) Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am J Physiol Cell Physiol 284(2):C571-581. https://doi.org/10.1152/ajpcell.00286.2002
Sun X, Zhou R, Lei Y, Hu J, Li X (2020) The ligand-gated ion channel P2X7 receptor mediates NLRP3/caspase-1-mediated pyroptosis in cerebral cortical neurons of juvenile rats with sepsis. Brain Res 1748:147109. https://doi.org/10.1016/j.brainres.2020.147109
Lin HY, Tang HY, Davis FB, Davis PJ (2011) Resveratrol and apoptosis. Ann N Y Acad Sci 1215:79–88. https://doi.org/10.1111/j.1749-6632.2010.05846.x
Lee HJ, Oh SY, Jo I (2021) Zearalenone induces endothelial cell apoptosis through activation of a cytosolic Ca(2+)/ERK1/2/p53/Caspase 3 Signaling Pathway. Toxins (Basel) 13(3). https://doi.org/10.3390/toxins13030187
Feng D, Wang B, Ma Y, Shi W, Tao K, Zeng W, Cai Q, Zhang Z et al (2016) The Ras/Raf/Erk pathway mediates the subarachnoid hemorrhage-induced apoptosis of hippocampal neurons through phosphorylation of p53. Mol Neurobiol 53(8):5737–5748. https://doi.org/10.1007/s12035-015-9490-x
Li C, Lönn ME, Xu X, Maghzal GJ, Frazer DM, Thomas SR, Halliwell B, Richardson DR et al (2012) Sustained expression of heme oxygenase-1 alters iron homeostasis in nonerythroid cells. Free Radic Biol Med 53(2):366–374. https://doi.org/10.1016/j.freeradbiomed.2012.03.007
Li J, Lu K, Sun F, Tan S, Zhang X, Sheng W, Hao W, Liu M et al (2021) Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J Transl Med 19(1):96. https://doi.org/10.1186/s12967-021-02745-1
Li S, Zhou C, Zhu Y, Chao Z, Sheng Z, Zhang Y, Zhao Y (2021) Ferrostatin-1 alleviates angiotensin II (Ang II)- induced inflammation and ferroptosis in astrocytes. Int Immunopharmacol 90:107179. https://doi.org/10.1016/j.intimp.2020.107179
Lv Z, Wang F, Zhang X, Zhang X, Zhang J, Liu R (2021) Etomidate attenuates the ferroptosis in myocardial ischemia/reperfusion rat model via Nrf2/HO-1 pathway. Shock 56(3):440–449. https://doi.org/10.1097/SHK.0000000000001751
Chang LC, Chiang SK, Chen SE, Yu YL, Chou RH, Chang WC (2018) Heme oxygenase-1 mediates BAY 11–7085 induced ferroptosis. Cancer Lett 416:124–137. https://doi.org/10.1016/j.canlet.2017.12.025
Fernández-Mendívil C, Luengo E, Trigo-Alonso P, García-Magro N, Negredo P, López MG (2021) Protective role of microglial HO-1 blockade in aging: implication of iron metabolism. Redox Biol 38:101789. https://doi.org/10.1016/j.redox.2020.101789
Tang Z, Ju Y, Dai X, Ni N, Liu Y, Zhang D, Gao H, Sun H et al (2021) HO-1-mediated ferroptosis as a target for protection against retinal pigment epithelium degeneration. Redox Biol 43:101971. https://doi.org/10.1016/j.redox.2021.101971
Wu A, Feng B, Yu J, Yan L, Che L, Zhuo Y, Luo Y, Yu B et al (2021) Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis. Redox Biol 46:102131. https://doi.org/10.1016/j.redox.2021.102131
Feng YD, Ye W, Tian W, Meng JR, Zhang M, Sun Y, Zhang HN, Wang SJ et al (2022) Old targets, new strategy: apigenin-7-O-β-d-(-6″-p-coumaroyl)-glucopyranoside prevents endothelial ferroptosis and alleviates intestinal ischemia-reperfusion injury through HO-1 and MAO-B inhibition. Free Radic Biol Med. https://doi.org/10.1016/j.freeradbiomed.2022.03.033
Ozen M, Kitase Y, Vasan V, Burkhardt C, Ramachandra S, Robinson S, Jantzie LL (2021) Chorioamnionitis precipitates perinatal alterations of heme-oxygenase-1 (HO-1) homeostasis in the developing rat brain. Int J Mol Sci 22(11). https://doi.org/10.3390/ijms22115773
Zhao J, Zhao X, Tian J, Xue R, Luo B, Lv J, Gao J, Wang M (2020) Theanine attenuates hippocampus damage of rat cerebral ischemia-reperfusion injury by inhibiting HO-1 expression and activating ERK1/2 pathway. Life Sci 241:117160. https://doi.org/10.1016/j.lfs.2019.117160
Acknowledgements
We are grateful to Wei Wang at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology for the experimental advice.
Funding
This article was supported by grants from the National Natural Science Foundation of China (Nos. 81971870 and No. 82172173).
Author information
Authors and Affiliations
Contributions
CL-L and QT designed and complete the study, conducted data analysis, and prepared the manuscript. JF-W, PB–H, YJ-G, CY, GJ-W, SM-H, and HW built MCAO models of rats and cultured HUVECs. MC-L reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All institutional and national guidelines for the care and use of laboratory animals were followed during the experiments. All procedures performed in this study observed the ethical standards of the Renmin Hospital of Wuhan University.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Tian, Q., Wang, J. et al. Blocking P2RX7 Attenuates Ferroptosis in Endothelium and Reduces HG-induced Hemorrhagic Transformation After MCAO by Inhibiting ERK1/2 and P53 Signaling Pathways. Mol Neurobiol 60, 460–479 (2023). https://doi.org/10.1007/s12035-022-03092-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03092-y