Abstract
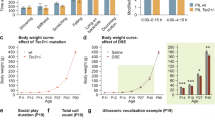
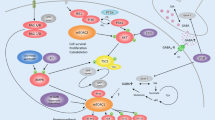
The mechanistic target of the rapamycin (mTOR) pathway is involved in cortical development. However, the efficacy of mTOR inhibitors in malformations of cortical dysplasia (MCD) outside of the tuberous sclerosis complex is unknown. We selected the MCD rat model with prenatal MAM exposure to test the efficacy of mTOR inhibitors in MCDs. We explored the early cortical changes of mTOR pathway protein expression in rats aged P15. We also monitored the early treatment effect of the mTOR inhibitor, rapamycin, on N-methyl-D-aspartate (NMDA)-induced spasms at P15 and their behavior in the juvenile stage. In vivo MR spectroscopy was performed after rapamycin treatment and compared with vehicle controls. There was no difference in mTORC1 pathway protein expression between MAM-exposed MCD rats and controls at P15, and prolonged treatment of rapamycin had no impact on NMDA-induced spasms despite poor weight gain. Prenatal MAM-exposed juvenile rats treated with rapamycin showed increased social approaching and freezing behavior during habituation. MR spectroscopy showed altered neurometabolites, including Gln, Glu+Gln, Tau, and Cr. Despite behavioral changes and in vivo neurometabolic alteration with early prolonged rapamycin treatment, rapamycin had no effect on spasms susceptibility in prenatal MAM-exposed infantile rats with MCD without mTORC1 activation. For MAM-exposed MCD rats without mTORC1 activation, treatment options outside of mTOR pathway inhibitors should be explored.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Iffland PH, Crino PB (2017) Focal cortical dysplasia: gene mutations, cell signaling, and therapeutic implications. Annu Rev Pathol 12:547–571. https://doi.org/10.1146/annurev-pathol-052016-100138
Crino PB (2019) Mechanistic target of rapamycin (mTOR) signaling in status epilepticus. Epilepsy Behav 101(Pt B):106550. https://doi.org/10.1016/j.yebeh.2019.106550
Crino PB (2016) The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol 12(7):379–392. https://doi.org/10.1038/nrneurol.2016.81
Griffith JL, Wong M (2018) The mTOR pathway in treatment of epilepsy: a clinical update. Future Neurol 13(2):49–58. https://doi.org/10.2217/fnl-2018-0001
Weston MC, Chen H, Swann JW (2012) Multiple roles for mammalian target of rapamycin signaling in both glutamatergic and GABAergic synaptic transmission. J Neurosci 32(33):11441–11452. https://doi.org/10.1523/JNEUROSCI.1283-12.2012
Buffington SA, Huang W, Costa-Mattioli M (2014) Translational control in synaptic plasticity and cognitive dysfunction. Annu Rev Neurosci 37(1):17–38. https://doi.org/10.1146/annurev-neuro-071013-014100
Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M et al (2010) Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis 40(1):193–199. https://doi.org/10.1016/j.nbd.2010.05.024
Zeng L-H, Rensing NR, Wong M (2009) The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci 29(21):6964–6972. https://doi.org/10.1523/jneurosci.0066-09.2009
Chachua T, Poon KL, Yum MS, Nesheiwat L, DeSantis K, Veliskova J, Velisek L (2012) Rapamycin has age-, treatment paradigm-, and model-specific anticonvulsant effects and modulates neuropeptide Y expression in rats. Epilepsia 53(11):2015–2025. https://doi.org/10.1111/j.1528-1167.2012.03674.x
Chevassus-Au-Louis N, Congar P, Represa A, Ben-Ari Y, Gaiarsa JL (1998) Neuronal migration disorders: heterotopic neocortical neurons in CA1 provide a bridge between the hippocampus and the neocortex. Proc Natl Acad Sci U S A 95(17):10263–10268
Germano IM, Sperber EF (1997) Increased seizure susceptibility in adult rats with neuronal migration disorders. Brain Res 777(1-2):219–222
Paredes M, Pleasure SJ, Baraban SC (2006) Embryonic and early postnatal abnormalities contributing to the development of hippocampal malformations in a rodent model of dysplasia. J Comp Neurol 495(1):133–148. https://doi.org/10.1002/cne.20871
Colacitti C, Sancini G, DeBiasi S, Franceschetti S, Caputi A, Frassoni C, Cattabeni F, Avanzini G et al (1999) Prenatal methylazoxymethanol treatment in rats produces brain abnormalities with morphological similarities to human developmental brain dysgeneses. J Neuropathol Exp Neurol 58(1):92–106
Crino PB (2015) Focal cortical dysplasia. Semin Neurol 35(3):201–208. https://doi.org/10.1055/s-0035-1552617
Choi BH, Lapham LW, Amin-Zaki L, Saleem T (1978) Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: a major effect of methylmercury poisoning in utero. J Neuropathol Exp Neurol 37(6):719–733. https://doi.org/10.1097/00005072-197811000-00001
Lombroso CT (2000) Can early postnatal closed head injury induce cortical dysplasia. Epilepsia 41(2):245–253. https://doi.org/10.1111/j.1528-1157.2000.tb00148.x
Lee M, Kim EJ, Woo DC, Shim WH, Yum MS (2020) In vivo MRI successfully reveals the malformation of cortical development in infant rats. Front Neurosci 14:510. https://doi.org/10.3389/fnins.2020.00510
Kim EH, Yum MS, Lee M, Kim EJ, Shim WH, Ko TS (2017) A new rat model of epileptic spasms based on methylazoxymethanol-induced malformations of cortical development. Front Neurol 8:271. https://doi.org/10.3389/fneur.2017.00271
Yum MS, Lee M, Ko TS, Velisek L (2014) A potential effect of ganaxolone in an animal model of infantile spasms. Epilepsy Res 108(9):1492–1500. https://doi.org/10.1016/j.eplepsyres.2014.08.015
Goosens KA, Maren S (2001) Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem 8(3):148–155. https://doi.org/10.1101/lm.37601
Marsan E, Baulac S (2018) Mechanistic target of rapamycin (mTOR) pathway, focal cortical dysplasia and epilepsy. Neuropathol Appl Neurobiol 44(1):6–17. https://doi.org/10.1111/nan.12463
Majolo F, Marinowic DR, Machado DC, Da Costa JC (2018) MTOR pathway in focal cortical dysplasia type 2: what do we know? Epilepsy Behav 85:157–163. https://doi.org/10.1016/j.yebeh.2018.05.014
Yao K, Duan Z, Zhou J, Li L, Zhai F, Dong Y, Wang X, Ma Z et al (2016) Clinical and immunohistochemical characteristics of type II and type I focal cortical dysplasia. Oncotarget 7(47):76415–76422. https://doi.org/10.18632/oncotarget.13001
Jansen LA, Mirzaa GM, Ishak GE, O'Roak BJ, Hiatt JB, Roden WH, Gunter SA, Christian SL et al (2015) PI3K/AKT pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain 138(Pt 6):1613–1628. https://doi.org/10.1093/brain/awv045
Kumari K, Sharma MC, Kakkar A, Malgulwar PB, Pathak P, Suri V, Sarkar C, Chandra SP et al (2020) mTOR pathway activation in focal cortical dysplasia. Ann Diagn Pathol 46:151523. https://doi.org/10.1016/j.anndiagpath.2020.151523
Carnero A, Paramio JM (2014) The PTEN/PI3K/AKT pathway in vivo, cancer mouse models. Front Oncol 4. https://doi.org/10.3389/fonc.2014.00252
Vazquez F, Ramaswamy S, Nakamura N, Sellers WR (2000) Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol 20(14):5010–5018. https://doi.org/10.1128/MCB.20.14.5010-5018.2000
Kielbinski M, Setkowicz Z, Gzielo K, Janeczko K (2018) Profiles of gene expression in the hippocampal formation of rats with experimentally-induced brain dysplasia. Dev Neurobiol 78(7):718–735. https://doi.org/10.1002/dneu.22595
Jesus-Ribeiro J, Pires LM, Melo JD, Ribeiro IP, Rebelo O, Sales F, Freire A, Melo JB (2020) Genomic and epigenetic advances in focal cortical dysplasia types I and II: a scoping review. Front Neurosci 14:580357. https://doi.org/10.3389/fnins.2020.580357
Hsieh LS, Wen JH, Claycomb K, Huang Y, Harrsch FA, Naegele JR, Hyder F, Buchanan GF et al (2016) Convulsive seizures from experimental focal cortical dysplasia occur independently of cell misplacement. Nat Commun 7:11753. https://doi.org/10.1038/ncomms11753
Lim JS, Kim WI, Kang HC, Kim SH, Park AH, Park EK, Cho YW, Kim S et al (2015) Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med 21(4):395–400. https://doi.org/10.1038/nm.3824
D'Gama AM, Poduri A (2021) Precision therapy for epilepsy related to brain malformations. Neurotherapeutics. https://doi.org/10.1007/s13311-021-01122-6
Theilmann W, Gericke B, Schidlitzki A, Muneeb Anjum SM, Borsdorf S, Harries T, Roberds SL, Aguiar DJ et al (2020) Novel brain permeant mTORC1/2 inhibitors are as efficacious as rapamycin or everolimus in mouse models of acquired partial epilepsy and tuberous sclerosis complex. Neuropharmacology 180:108297. https://doi.org/10.1016/j.neuropharm.2020.108297
Talos DM, Sun H, Kosaras B, Joseph A, Folkerth RD, Poduri A, Madsen JR, Black PM et al (2012) Altered inhibition in tuberous sclerosis and type IIb cortical dysplasia. Ann Neurol 71(4):539–551. https://doi.org/10.1002/ana.22696
Ruffolo G, Iyer A, Cifelli P, Roseti C, Muhlebner A, van Scheppingen J, Scholl T, Hainfellner JA et al (2016) Functional aspects of early brain development are preserved in tuberous sclerosis complex (TSC) epileptogenic lesions. Neurobiol Dis 95:93–101. https://doi.org/10.1016/j.nbd.2016.07.014
Cepeda C, Levinson S, Yazon VW, Barry J, Mathern GW, Fallah A, Vinters HV, Levine MS, Wu JY (2018) Cellular antiseizure mechanisms of everolimus in pediatric tuberous sclerosis complex, cortical dysplasia, and non-mTOR-mediated etiologies. Epilepsia Open 3(Suppl Suppl 2):180–190. https://doi.org/10.1002/epi4.12253
Ringstedt T, Linnarsson S, Wagner J, Lendahl U, Kokaia Z, Arenas E, Ernfors P, Ibáñez CF (1998) BDNF regulates reelin expression and Cajal-Retzius cell development in the cerebral cortex. Neuron 21(2):305–315. https://doi.org/10.1016/s0896-6273(00)80540-1
Lizarraga SB, Ma L, Maguire AM, van Dyck LI, Wu Q, Ouyang Q, Kavanaugh BC, Nagda D et al (2021) Human neurons from Christianson syndrome iPSCs reveal mutation-specific responses to rescue strategies. Sci Transl Med 13(580). https://doi.org/10.1126/scitranslmed.aaw0682
Zheng DH, Guo W, Sun FJ, Xu GZ, Zang ZL, Shu HF, Yang H (2016) Expression of TRPC6 and BDNF in cortical lesions from patients with focal cortical dysplasia. J Neuropathol Exp Neurol 75(8):718–730. https://doi.org/10.1093/jnen/nlw044
Ku KM, Weir RK, Silverman JL, Berman RF, Bauman MD (2016) Behavioral phenotyping of juvenile Long-Evans and Sprague-Dawley rats: implications for preclinical models of autism spectrum disorders. PLoS One 11(6):e0158150. https://doi.org/10.1371/journal.pone.0158150
Tkac I, Rao R, Georgieff MK, Gruetter R (2003) Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn Reson Med 50(1):24–32. https://doi.org/10.1002/mrm.10497
Ripps H, Shen W (2012) Review: taurine: a “very essential” amino acid. Mol Vis 18:2673–2686
Oja SS, Saransaari P (2013) Taurine and epilepsy. Epilepsy Res 104(3):187–194. https://doi.org/10.1016/j.eplepsyres.2013.01.010
Rodrigo R, Felipo V (2007) Control of brain glutamine synthesis by NMDA receptors. Front Biosci 12:883–890. https://doi.org/10.2741/2110
Blüml S, Wisnowski JL, Nelson MD, Paquette L, Gilles FH, Kinney HC, Panigrahy A (2013) Metabolic maturation of the human brain from birth through adolescence: insights from in vivo magnetic resonance spectroscopy. Cereb Cortex 23(12):2944–2955. https://doi.org/10.1093/cercor/bhs283
Towner RA, Gulej R, Zalles M, Saunders D, Smith N, Lerner M, Morton KA, Richardson A (2021) Rapamycin restores brain vasculature, metabolism, and blood-brain barrier in an inflammaging model. Geroscience 43(2):563–578. https://doi.org/10.1007/s11357-021-00363-9
Morgan JJ, Kleven GA, Tulbert CD, Olson J, Horita DA, Ronca AE (2013) Longitudinal 1H MRS of rat forebrain from infancy to adulthood reveals adolescence as a distinctive phase of neurometabolite development. NMR Biomed 26(6):683–691. https://doi.org/10.1002/nbm.2913
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1A2C100447111) and a grant IL0006 from Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea. The Basic Science Research Program supported M.L. through the NRF funded by the Ministry of Education (NRF-2019R1A6A3A01090600).
Author information
Authors and Affiliations
Contributions
M.-S.Y. contributed to the conception and design of the study; M.L. and E.-J.K. contributed to the acquisition of data; M.L. and E.-J.K. contributed to the analysis of data; M.L., E.-J.K., and M.-S.Y. contributed to drafting and editing the manuscript and figures; M.L., M.-J Kim, and M.-S.Y. reviewed the submitted version of the manuscript and supervised the study.
Corresponding author
Ethics declarations
Ethics Approval
All experiments were approved by the Institutional Animal Care and Use Committee of the Ulsan University College of Medicine and conducted in accordance with the revised Guide for the Care and Use of Laboratory Animals [NIH GUIDE, 8th Edition, 2011].
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information

Supplementary Table 1
(PNG 342 kb)

Supplementary Fig. 1
MAM-exposed rats showed increased time in zone with strangers (CSR, control vs. MAM; 375.58±100.04 vs 470.44±224.03, p=0.016) but decreased time in empty cage (EC, 133.91±62.08 vs 65.79±170.51, p=0.004) (A) MAM-exposed rats also showed reduced entries to both cage (CSR, 10.36±7.10 vs 1.08±2.02, p=0.000; EC, 9.27±6.74 vs 0.58±1.0, p=0.000)in sociability test suggesting increased sociability (B) and there was no significant difference of freezing activity (4.76±3.71 vs 10.2±9.74, p=0.236) between MAM-exposed rats and controls in baseline fear conditioning (C) (PNG 223 kb)

Supplementary Table 2
(PNG 527 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, M., Kim, EJ., Kim, MJ. et al. Rapamycin Cannot Reduce Seizure Susceptibility in Infantile Rats with Malformations of Cortical Development Lacking mTORC1 Activation. Mol Neurobiol 59, 7439–7449 (2022). https://doi.org/10.1007/s12035-022-03033-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03033-9