Abstract
Spinal cord injury (SCI) can result in significant neurological impairment and functional and cognitive deficits. It is well established that SCI results in focal neurodegeneration that gradually spreads to other cord areas. On the other hand, traumatic brain injury (TBI) is strongly associated with tau protein pathology and neurodegeneration that can spread in areas throughout the brain. Tau is a microtubule-associated protein abundant in neurons and whose abnormalities result in neuronal cell death. While SCI and TBI have been extensively studied, there is limited research on the relationship between SCI and brain tau pathology. As a result, in this study, we examined tau pathology in spinal cord and brain samples obtained from severe SCI mouse models at various time points. The effects of severe SCI on locomotor function, spatial memory, anxiety/risk-taking behavior were investigated. Immunostaining and immunoblotting confirmed a progressive increase in tau pathology in the spinal cord and brain areas. Moreover, we used electron microscopy to examine brain samples and observed disrupted mitochondria and microtubule structure following SCI. SCI resulted in motor dysfunction, memory impairment, and abnormal risk-taking behavior. Notably, eliminating pathogenic cis P-tau via systemic administration of appropriate monoclonal antibodies restored SCI’s pathological and functional consequences. Thus, our findings suggest that SCI causes severe tauopathy that spreads to brain areas, indicating brain dysfunction. Additionally, tau immunotherapy with an anti-cis P-tau antibody could suppress pathogenic outcomes in SCI mouse models, with significant clinical implications for SCI patients.
Graphical abstract
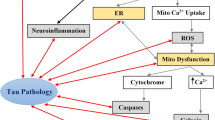
SCI induces profound pathogenic cis p-tau, which diffuses into the brain through CSF, resulting in brain neurodegeneration and cognitive decline.






Similar content being viewed by others
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SCI:
-
Spinal cord injury
- CSF:
-
Cerebrospinal fluid
- TBI:
-
Traumatic brain injury
- MTs:
-
Microtubules
- CTE:
-
Chronic traumatic encephalography
- cis P-tau:
-
cis PThr231-tau
- AD:
-
Alzheimer’s disease
- Pin1:
-
Peptidyl-prolyl cis/trans isomerase
- Cis mAb:
-
cis P-tau monoclonal antibody
- sSCI:
-
Severe SCI
- NIH:
-
National Institute of Health
- SC:
-
Subcutaneously
- BMS:
-
The Basso mouse scale
- OF:
-
Open-field
- IP:
-
Intraperitoneal
- TEM:
-
Transmission electron microscopy
- EPM:
-
Elevated plus-maze
- ROI:
-
Region of interest
- ANOVA:
-
Analysis of variance
- SD:
-
Standard deviation
- ssTBI:
-
Single severe TBI
- rmTBI:
-
Repetitive mild TBI
- LTP:
-
Long-term potentiation
References
Ackery A, Tator C, Krassioukov A (2004) A global perspective on spinal cord injury epidemiology. J Neurotrauma 21(10):1355–1370. https://doi.org/10.1089/neu.2004.21.1355
Van den Berg ME, Castellote JM, Mahillo-Fernandez I, de Pedro-Cuesta J (2010) Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology 34(3):184–192. https://doi.org/10.1159/000279335
Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG (2014) Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol 6:309–331. https://doi.org/10.2147/CLEP.S68889
Collins-Praino LE, Corrigan F (2017) Does neuroinflammation drive the relationship between tau hyperphosphorylation and dementia development following traumatic brain injury? Brain Behav Immun 60:369–382. https://doi.org/10.1016/j.bbi.2016.09.027
Bethea JR, Dietrich WD (2002) Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol 15(3):355–360. https://doi.org/10.1097/00019052-200206000-00021
Tator CH, Fehlings MG (1991) Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 75(1):15–26. https://doi.org/10.3171/jns.1991.75.1.0015
Nielson JL, Sears-Kraxberger I, Strong MK, Wong JK, Willenberg R, Steward O (2010) Unexpected survival of neurons of origin of the pyramidal tract after spinal cord injury. J Neurosci 30(34):11516–11528. https://doi.org/10.1523/JNEUROSCI.1433-10.2010
Coulthart MB, Jansen GH, Cashman NR (2016) Evidence for transmissibility of Alzheimer disease pathology: Cause for concern? CMAJ 188(10):E210–E212. https://doi.org/10.1503/cmaj.151257
Craig A, Guest R, Tran Y, Middleton J (2017) Cognitive Impairment and Mood States after Spinal Cord Injury. J Neurotrauma 34(6):1156–1163. https://doi.org/10.1089/neu.2016.4632
Nielson JL, Strong MK, Steward O (2011) A reassessment of whether cortical motor neurons die following spinal cord injury. J Comp Neurol 519(14):2852–2869. https://doi.org/10.1002/cne.22661
Wannier T, Schmidlin E, Bloch J, Rouiller EM (2005) A unilateral section of the corticospinal tract at cervical level in primate does not lead to measurable cell loss in motor cortex. J Neurotrauma 22(6):703–717. https://doi.org/10.1089/neu.2005.22.703
Cohen ML, Tulsky DS, Holdnack JA, Carlozzi NE, Wong A, Magasi S, Heaton RK, Heinemann AW (2017) Cognition among community-dwelling individuals with spinal cord injury. Rehabil Psychol 62(4):425–434. https://doi.org/10.1037/rep0000140
Jeter CB, Hergenroeder GW, Hylin MJ, Redell JB, Moore AN, Dash PK (2013) Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J Neurotrauma 30(8):657–670. https://doi.org/10.1089/neu.2012.2439
Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K (1994) Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci U S A 91(12):5562–5566. https://doi.org/10.1073/pnas.91.12.5562
Gendron TF, Petrucelli L (2009) The role of tau in neurodegeneration. Mol Neurodegener 4:13. https://doi.org/10.1186/1750-1326-4-13
Iqbal K, Liu F, Gong CX (2016) Tau and neurodegenerative disease: the story so far. Nat Rev Neurol 12(1):15–27. https://doi.org/10.1038/nrneurol.2015.225
Wang Y, Mandelkow E (2016) Tau in physiology and pathology. Nat Rev Neurosci 17(1):5–21. https://doi.org/10.1038/nrn.2015.1
Blennow K, Hardy J, Zetterberg H (2012) The neuropathology and neurobiology of traumatic brain injury. Neuron 76(5):886–899. https://doi.org/10.1016/j.neuron.2012.11.021
DeKosky ST, Blennow K, Ikonomovic MD, Gandy S (2013) Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat Rev Neurol 9(4):192–200. https://doi.org/10.1038/nrneurol.2013.36
McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, Lee HS, Wojtowicz SM, Hall G, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136(Pt 1):43–64. https://doi.org/10.1093/brain/aws307
Smith DH, Johnson VE, Stewart W (2013) Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 9(4):211–221. https://doi.org/10.1038/nrneurol.2013.29
Zare-Shahabadi A, Masliah E, Johnson GV, Rezaei N (2015) Autophagy in Alzheimer’s disease. Rev Neurosci 26(4):385–395. https://doi.org/10.1515/revneuro-2014-0076
Chen CH, Li W, Sultana R, You MH, Kondo A, Shahpasand K, Kim BM, Luo ML, Nechama M, Lin YM, Yao Y, Lee TH, Zhou XZ, Swomley AM, Butterfield DA, Zhang Y, Lu KP (2015) Pin1 cysteine-113 oxidation inhibits its catalytic activity and cellular function in Alzheimer’s disease. Neurobiol Dis 76:13–23. https://doi.org/10.1016/j.nbd.2014.12.027
Lee TH, Chen CH, Suizu F, Huang P, Schiene-Fischer C, Daum S, Zhang YJ, Goate A, Chen RH, Zhou XZ, Lu KP (2011) Death-associated protein kinase 1 phosphorylates Pin1 and inhibits its prolyl isomerase activity and cellular function. Mol Cell 42(2):147–159. https://doi.org/10.1016/j.molcel.2011.03.005
Lim J, Balastik M, Lee TH, Nakamura K, Liou YC, Sun A, Finn G, Pastorino L, Lee VM, Lu KP (2008) Pin1 has opposite effects on wild-type and P301L tau stability and tauopathy. J Clin Invest 118(5):1877–1889. https://doi.org/10.1172/JCI34308
Liou YC, Sun A, Ryo A, Zhou XZ, Yu ZX, Huang HK, Uchida T, Bronson R, Bing G, Li X, Hunter T, Lu KP (2003) Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature 424(6948):556–561. https://doi.org/10.1038/nature01832
Lu KP, Hanes SD, Hunter T (1996) A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 380(6574):544–547. https://doi.org/10.1038/380544a0
Lu KP, Zhou XZ (2007) The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol 8(11):904–916. https://doi.org/10.1038/nrm2261
Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP (1999) The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 399(6738):784–788. https://doi.org/10.1038/21650
Pastorino L, Sun A, Lu PJ, Zhou XZ, Balastik M, Finn G, Wulf G, Lim J, Li SH, Li X, Xia W, Nicholson LK, Lu KP (2006) The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-beta production. Nature 440(7083):528–534. https://doi.org/10.1038/nature04543
Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Markesbery WR, Zhou XZ, Lu KP, Butterfield DA (2006) Oxidative modification and down-regulation of Pin1 in Alzheimer’s disease hippocampus: A redox proteomics analysis. Neurobiol Aging 27(7):918–925. https://doi.org/10.1016/j.neurobiolaging.2005.05.005
Roqanian S, Ahmadian S, Nabavi SM, Pakdaman H, Shafiezadeh M, Goudarzi G, Shahpasand K (2022) Tau nuclear translocation is a leading step in tau pathology process through P53 stabilization and nucleolar dispersion. J Neurosci Res. https://doi.org/10.1002/jnr.25024
Albayram O, Kondo A, Mannix R, Smith C, Tsai CY, Li C, Herbert MK, Qiu J, Monuteaux M, Driver J, Yan S, Gormley W, Puccio AM, Okonkwo DO, Lucke-Wold B, Bailes J, Meehan W, Zeidel M, Lu KP, Zhou XZ (2017) Cis P-tau is induced in clinical and preclinical brain injury and contributes to post-injury sequelae. Nat Commun 8(1):1000. https://doi.org/10.1038/s41467-017-01068-4
Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH, Yao Y, Lin YM, Driver JA, Sun Y, Wei S, Luo ML, Albayram O, Huang P, Rotenberg A, Ryo A, Goldstein LE, Pascual-Leone A, McKee AC, Meehan W, Zhou XZ, Lu KP (2015) Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 523(7561):431–436. https://doi.org/10.1038/nature14658
Lu KP, Kondo A, Albayram O, Herbert MK, Liu H, Zhou XZ (2016) Potential of the Antibody Against cis-Phosphorylated Tau in the Early Diagnosis, Treatment, and Prevention of Alzheimer Disease and Brain Injury. JAMA Neurol 73(11):1356–1362. https://doi.org/10.1001/jamaneurol.2016.2027
Nakamura K, Greenwood A, Binder L, Bigio EH, Denial S, Nicholson L, Zhou XZ, Lu KP (2012) Proline isomer-specific antibodies reveal the early pathogenic tau conformation in Alzheimer’s disease. Cell 149(1):232–244. https://doi.org/10.1016/j.cell.2012.02.016
Albayram O, Herbert MK, Kondo A, Tsai CY, Baxley S, Lian X, Hansen M, Zhou XZ, Lu KP (2016) Function and regulation of tau conformations in the development and treatment of traumatic brain injury and neurodegeneration. Cell Biosci 6:59. https://doi.org/10.1186/s13578-016-0124-4
Nakhjiri E, Vafaee MS, Hojjati SMM, Shahabi P, Shahpasand K (2020) Tau Pathology Triggered by Spinal Cord Injury Can Play a Critical Role in the Neurotrauma Development. Mol Neurobiol 57(11):4845–4855. https://doi.org/10.1007/s12035-020-02061-7
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24(9):2143–2155. https://doi.org/10.1523/JNEUROSCI.3547-03.2004
Plemel JR, Duncan G, Chen KW, Shannon C, Park S, Sparling JS, Tetzlaff W (2008) A graded forceps crush spinal cord injury model in mice. J Neurotrauma 25(4):350–370. https://doi.org/10.1089/neu.2007.0426
Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG (2006) Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23(5):635–659. https://doi.org/10.1089/neu.2006.23.635
Wu J, Stoica BA, Luo T, Sabirzhanov B, Zhao Z, Guanciale K, Nayar SK, Foss CA, Pomper MG, Faden AI (2014) Isolated spinal cord contusion in rats induces chronic brain neuroinflammation, neurodegeneration, and cognitive impairment Involvement of cell cycle activation. Cell Cycle 13(15):2446–2458. https://doi.org/10.4161/cc.29420
De Risi M, Tufano M, Alvino FG, Ferraro MG, Torromino G, Gigante Y, Monfregola J, Marrocco E, Pulcrano S, Tunisi L, Lubrano C, Papy-Garcia D, Tuchman Y, Salleo A, Santoro F, Bellenchi GC, Cristino L, Ballabio A, Fraldi A, De Leonibus E (2021) Altered heparan sulfate metabolism during development triggers dopamine-dependent autistic-behaviours in models of lysosomal storage disorders. Nat Commun 12(1):3495. https://doi.org/10.1038/s41467-021-23903-5
Hefner K, Cameron HA, Karlsson RM, Holmes A (2007) Short-term and long-term effects of postnatal exposure to an adult male in C57BL/6J mice. Behav Brain Res 182(2):344–348. https://doi.org/10.1016/j.bbr.2007.03.032
Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L, Vishwanath J, Saavedra MC, Innerfield CE, Jacoby AS, Shine J, Iismaa TP, Crawley JN (2003) Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology 28(6):1031–1044. https://doi.org/10.1038/sj.npp.1300164
Wu J, Zhao Z, Sabirzhanov B, Stoica BA, Kumar A, Luo T, Skovira J, Faden AI (2014) Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci 34(33):10989–11006. https://doi.org/10.1523/JNEUROSCI.5110-13.2014
Adhikari A, Topiwala MA, Gordon JA (2011) Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron 71(5):898–910. https://doi.org/10.1016/j.neuron.2011.07.027
Gholami M, Saboory E, Khalkhali HR (2014) Chronic morphine and tramadol re-exposure induced an anti-anxiety effect in prepubertal rats exposed neonatally to the same drugs. Clin Exp Pharmacol Physiol 41(10):838–843. https://doi.org/10.1111/1440-1681.12274
Hogg S (1996) A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav 54(1):21–30. https://doi.org/10.1016/0091-3057(95)02126-4
Korte SM, De Boer SF (2003) A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol 463(1–3):163–175. https://doi.org/10.1016/s0014-2999(03)01279-2
Nakhjiri E, Saboory E, Roshan-Milani S, Rasmi Y, Khalafkhani D (2017) Effect of prenatal restraint stress and morphine co-administration on plasma vasopressin concentration and anxiety behaviors in adult rat offspring. Stress 20(2):205–211. https://doi.org/10.1080/10253890.2017.1306053
Rodgers RJ, Dalvi A (1997) Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev 21(6):801–810. https://doi.org/10.1016/s0149-7634(96)00058-9
Schindowski K, Bretteville A, Leroy K, Begard S, Brion JP, Hamdane M, Buee L (2006) Alzheimer’s disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am J Pathol 169(2):599–616. https://doi.org/10.2353/ajpath.2006.060002
Baratz R, Rubovitch V, Frenk H, Pick CG (2010) The influence of alcohol on behavioral recovery after mTBI in mice. J Neurotrauma 27(3):555–563. https://doi.org/10.1089/neu.2009.0891
Sierksma AS, van den Hove DL, Pfau F, Philippens M, Bruno O, Fedele E, Ricciarelli R, Steinbusch HW, Vanmierlo T, Prickaerts J (2014) Improvement of spatial memory function in APPswe/PS1dE9 mice after chronic inhibition of phosphodiesterase type 4D. Neuropharmacology 77:120–130. https://doi.org/10.1016/j.neuropharm.2013.09.015
Pedersen JT, Sigurdsson EM (2015) Tau immunotherapy for Alzheimer’s disease. Trends Mol Med 21(6):394–402. https://doi.org/10.1016/j.molmed.2015.03.003
Rosenmann H (2013) Immunotherapy for targeting tau pathology in Alzheimer’s disease and tauopathies. Curr Alzheimer Res 10(3):217–228. https://doi.org/10.2174/1567205011310030001
Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O’Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A (2016) The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 537(7618):50–56. https://doi.org/10.1038/nature19323
Brody DL, Holtzman DM (2008) Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci 31:175–193. https://doi.org/10.1146/annurev.neuro.31.060407.125529
Qiu C, Albayram O, Kondo A, Wang B, Kim N, Arai K, Tsai CY, Bassal MA, Herbert MK, Washida K, Angeli P, Kozono S, Stucky JE, Baxley S, Lin YM, Sun Y, Rotenberg A, Caldarone BJ, Bigio EH, Chen X, Tenen DG, Zeidel M, Lo EH, Zhou XZ, Lu KP (2021) Cis P-tau underlies vascular contribution to cognitive impairment and dementia and can be effectively targeted by immunotherapy in mice. Sci Transl Med 13 (596). https://doi.org/10.1126/scitranslmed.aaz7615
Shahpasand K, Sepehri Shamloo A, Nabavi SM, Ping LuK, Zhen Zhou X (2018) Tau immunotherapy: Hopes and hindrances. Hum Vaccin Immunother 14(2):277–284. https://doi.org/10.1080/21645515.2017.1393594
Cavdar S, Onat FY, Cakmak YO, Yananli HR, Gulcebi M, Aker R (2008) The pathways connecting the hippocampal formation, the thalamic reuniens nucleus and the thalamic reticular nucleus in the rat. J Anat 212(3):249–256. https://doi.org/10.1111/j.1469-7580.2008.00858.x
Nardone R, Holler Y, Brigo F, Seidl M, Christova M, Bergmann J, Golaszewski S, Trinka E (2013) Functional brain reorganization after spinal cord injury: systematic review of animal and human studies. Brain Res 1504:58–73. https://doi.org/10.1016/j.brainres.2012.12.034
Hulsebosch CE, Hains BC, Crown ED, Carlton SM (2009) Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev 60(1):202–213. https://doi.org/10.1016/j.brainresrev.2008.12.010
Zhao P, Waxman SG, Hains BC (2007) Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci 27(33):8893–8902. https://doi.org/10.1523/JNEUROSCI.2209-07.2007
Ankeny DP, Popovich PG (2009) Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience 158(3):1112–1121. https://doi.org/10.1016/j.neuroscience.2008.07.001
Segal MB (1993) Extracellular and cerebrospinal fluids. J Inherit Metab Dis 16(4):617–638. https://doi.org/10.1007/BF00711896
Zetterberg H (2017) Review: Tau in biofluids - relation to pathology, imaging and clinical features. Neuropathol Appl Neurobiol 43(3):194–199. https://doi.org/10.1111/nan.12378
Shultz SR, Wright DK, Zheng P, Stuchbery R, Liu SJ, Sashindranath M, Medcalf RL, Johnston LA, Hovens CM, Jones NC, O’Brien TJ (2015) Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain 138(Pt 5):1297–1313. https://doi.org/10.1093/brain/awv053
Yokobori S, Zhang Z, Moghieb A, Mondello S, Gajavelli S, Dietrich WD, Bramlett H, Hayes RL, Wang M, Wang KK, Bullock MR (2015) Acute diagnostic biomarkers for spinal cord injury: review of the literature and preliminary research report. World Neurosurg 83(5):867–878. https://doi.org/10.1016/j.wneu.2013.03.012
Johnson VE, Stewart W, Smith DH (2013) Axonal pathology in traumatic brain injury. Exp Neurol 246:35–43. https://doi.org/10.1016/j.expneurol.2012.01.013
Funding
The grant of this study was provided by Neurosciences Research Center of Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran; Council for Stem Cell Sciences and Technologies, Tehran, Iran (Grant number: 11/35721); and grant #1397-A-5443 from Royan Institute for Stem Cell Biology & Technology, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Elnaz Nakhjiri expressed the idea of the article and performed the literature search. Material preparation, data collection and analysis were performed by Selva Zamanzadeh, Ehsan Ehsani, Hamid Soltani Zangbar, Shaqayeq Roqanian, Daryoush Mohammadnejad and Shahin Ahmadian. The first draft of the manuscript was written by Elnaz Nakhjiri, Koorosh Shahpasand and Parviz Shahabi. All authors edited the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was conducted following the Declaration of Helsinki’s principles. The Tabriz University of Medical Sciences Ethics Committee approved this study (Code of ethics: IR.TBZMED.VCR.REC.1398.067).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakhjiri, E., Roqanian, S., Zangbar, H.S. et al. Spinal Cord Injury Causes Prominent Tau Pathology Associated with Brain Post-Injury Sequela. Mol Neurobiol 59, 4197–4208 (2022). https://doi.org/10.1007/s12035-022-02843-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02843-1