Abstract
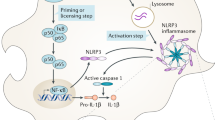
Inflammasomes are intracellular protein complexes, members of the innate immune system, and their activation and regulation play an essential role in maintaining homeostatic conditions against exogenous and endogenous stimuli. Inflammasomes occur as cytosolic proteins and assemble into a complex during the recognition of pathogen-associated or danger-associated molecular patterns by pattern-recognition receptors in host cells. The formation of the inflammasome complex elicits signaling molecules of proinflammatory cytokines such as interleukin-1β and interleukin 18 via activation of caspase-1 in the canonical inflammasome pathway whereas caspase-11 in the case of a mouse and caspase-4 and caspase-5 in the case of humans in the non-canonical inflammasome pathway, resulting in pyroptotic or inflammatory cell death which ultimately leads to neuroinflammation and neurodegenerative diseases. Inflammasome activation, particularly in microglial cells and macrophages, has been linked to aging as well as age-related neurodegenerative diseases. The accumulation of abnormal/ misfolded proteins acts as a ligand for inflammasome activation in neurodegenerative diseases. Although recent studies have revealed the inflammasomes’ functionality in both in vitro and in vivo models, many inflammasome signaling cascade activations during biological aging, neuroinflammation, and neurodegeneration are still ambiguous. In this review, we comprehensively unveil the cellular and molecular mechanisms of inflammasome activation during neuronal aging and age-related neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, prion disease, and amyotrophic lateral sclerosis.




Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- AIM2:
-
Absent-in-melanoma 2
- AD:
-
Alzheimer’s disease
- APP:
-
Amyloid-beta precursor protein
- ASC:
-
Apoptosis-associated speck-like protein containing a caspase-recruitment domain
- CCL3:
-
C-C motif chemokine ligand 3
- PrPc :
-
Cellular prion protein
- CSF:
-
Cerebrospinal fluid
- DAMP:
-
Damage-associated molecular pattern
- GSDMD:
-
Gasdermin D
- IFN:
-
Interferon
- IL:
-
Interleukin
- IL-1R:
-
IL-1 receptor
- LRR:
-
Leucine-rich repeat
- NFκB:
-
Nuclear factor kappa-light-chain-enhancer of activated B
- NLR:
-
Nod-like receptor
- NLRP:
-
Nod-like receptor protein
- NLRP3:
-
NLR family pyrin domain containing 3
- NLRC4:
-
NLR family CARD domain-containing protein 4
- NOD:
-
Nucleotide-binding oligomerization domain
- PAMP:
-
Pathogen-associated molecular pattern
- PRR:
-
Pattern-recognition receptor
- P2RX7:
-
P2X purinoceptor 7
- PYD:
-
Pyrin domain
- PrPsc :
-
Scrapie isoform of the prion protein
- TLR:
-
Toll-like receptor
- TLR4:
-
Toll-like receptor 4
- TNF:
-
Tumor necrosis factor
References
Ottis P, Koppe K, Onisko B, Dynin I, Arzberger T, Kretzschmar H, Requena JR, Silva CJ et al (2012) Human and rat brain lipofuscin proteome. Proteomics 12(15–16):2445–2454. https://doi.org/10.1002/pmic.201100668
Clewett DV, Lee TH, Greening S, Ponzio A, Margalit E, Mather M (2016) Neuromelanin marks the spot: identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol Aging 37:117–126. https://doi.org/10.1016/j.neurobiolaging.2015.09.019
Latz E, Duewell P (2018) NLRP3 inflammasome activation in inflammaging. Semin Immunol 40:61–73. https://doi.org/10.1016/j.smim.2018.09.001
Singh T, Newman AB (2011) Inflammatory markers in population studies of aging. Ageing Res Rev 10(3):319–329. https://doi.org/10.1016/j.arr.2010.11.002
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A (2018) Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14(10):576–590. https://doi.org/10.1038/s41574-018-0059-4
Franceschi C, Zaikin A, Gordleeva S, Ivanchenko M, Bonifazi F, Storci G, Bonafe M (2018) Inflammaging 2018: an update and a model. Semin Immunol 40:1–5. https://doi.org/10.1016/j.smim.2018.10.008
Calabrese V, Santoro A, Monti D, Crupi R, Di Paola R, Latteri S, Cuzzocrea S, Zappia M et al (2018) Aging and Parkinson’s disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radical Biol Med 115:80–91. https://doi.org/10.1016/j.freeradbiomed.2017.10.379
Cicolari S, Catapano AL, Magni P (2021) Inflammaging and neurodegenerative diseases: role of NLRP3 inflammasome activation in brain atherosclerotic vascular disease. Mech Ageing Dev 195:111467. https://doi.org/10.1016/j.mad.2021.111467
Dugger BN, Dickson DW (2017) Pathology of neurodegenerative diseases. Cold Spring Harbor perspectives in biology 9(7):a028035. https://doi.org/10.1101/cshperspect.a028035
Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19(10):610–621. https://doi.org/10.1038/s41583-018-0055-7
Kumar M, Roe K, Orillo B, Muruve DA, Nerurkar VR, Gale M Jr, Verma S (2013) Inflammasome adaptor protein apoptosis-associated speck-like protein containing CARD (ASC) is critical for the immune response and survival in west Nile virus encephalitis. J Virol 87(7):3655–3667. https://doi.org/10.1128/JVI.02667-12
Fink SL, Bergsbaken T, Cookson BT (2008) Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci U S A 105(11):4312–4317. https://doi.org/10.1073/pnas.0707370105
Khare S, Luc N, Dorfleutner A, Stehlik C (2010) Inflammasomes and their activation. Crit Rev Immunol 30(5):463–487. https://doi.org/10.1615/critrevimmunol.v30.i5.50
Guzman-Martinez L, Maccioni RB, Andrade V, Navarrete LP, Pastor MG, Ramos-Escobar N (2019) Neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol 10:1008. https://doi.org/10.3389/fphar.2019.01008
Yoneyama M, Fujita T (2008) Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity 29(2):178–181. https://doi.org/10.1016/j.immuni.2008.07.009
Benko S, Philpott DJ, Girardin SE (2008) The microbial and danger signals that activate Nod-like receptors. Cytokine 43(3):368–373. https://doi.org/10.1016/j.cyto.2008.07.013
Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA (2009) AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458(7237):514–518. https://doi.org/10.1038/nature07725
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13(6):397–411. https://doi.org/10.1038/nri3452
Hu MY, Lin YY, Zhang BJ, Lu DL, Lu ZQ, Cai W (2019) Update of inflammasome activation in microglia/macrophage in aging and aging-related disease. CNS Neurosci Ther 25(12):1299–1307. https://doi.org/10.1111/cns.13262
Mammana S, Fagone P, Cavalli E, Basile MS, Petralia MC, Nicoletti F, Bramanti P, Mazzon E (2018) The role of macrophages in neuroinflammatory and neurodegenerative pathways of Alzheimer's disease, amyotrophic lateral sclerosis, and multiple sclerosis: pathogenetic cellular effectors and potential therapeutic targets. Int J Mol Sci 19(3): 831. https://doi.org/10.3390/ijms19030831
Davis EJ, Foster TD, Thomas WE (1994) Cellular forms and functions of brain microglia. Brain Res Bull 34(1):73–78. https://doi.org/10.1016/0361-9230(94)90189-9
Marin-Aguilar F, Ruiz-Cabello J, Cordero MD (2018) Aging and the inflammasomes. Exp Suppl 108:303–320. https://doi.org/10.1007/978-3-319-89390-7_13
Mejias NH, Martinez CC, Stephens ME, de Rivero Vaccari JP (2018) Contribution of the inflammasome to inflammaging. J Inflamm (Lond) 15:23. https://doi.org/10.1186/s12950-018-0198-3
Youm YH, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S et al (2013) Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab 18(4):519–532. https://doi.org/10.1016/j.cmet.2013.09.010
Sebastian-Valverde M, Pasinetti GM (2020) The NLRP3 inflammasome as a critical actor in the inflammaging process. Cells 9(6):1552. https://doi.org/10.3390/cells9061552
Labzin LI, Heneka MT, Latz E (2018) Innate immunity and neurodegeneration. Annu Rev Med 69:437–449. https://doi.org/10.1146/annurev-med-050715-104343
Furman D, Jojic V, Kidd B, Shen-Orr S, Price J, Jarrell J, Tse T, Huang H et al (2013) Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol Syst Biol 9:659. https://doi.org/10.1038/msb.2013.15
Sardi F, Fassina L, Venturini L, Inguscio M, Guerriero F, Rolfo E, Ricevuti G (2011) Alzheimer’s disease, autoimmunity and inflammation. The good, the bad and the ugly. Autoimmun Rev 11(2):149–153. https://doi.org/10.1016/j.autrev.2011.09.005
Scrivo R, Vasile M, Bartosiewicz I, Valesini G (2011) Inflammation as “common soil” of the multifactorial diseases. Autoimmun Rev 10(7):369–374. https://doi.org/10.1016/j.autrev.2010.12.006
Wang WY, Tan MS, Yu JT, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3(10):136. https://doi.org/10.3978/j.issn.2305-5839.2015.03.49
Tha KK, Okuma Y, Miyazaki H, Murayama T, Uehara T, Hatakeyama R, Hayashi Y, Nomura Y (2000) Changes in expressions of proinflammatory cytokines IL-1beta, TNF-alpha and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res 885(1):25–31. https://doi.org/10.1016/s0006-8993(00)02883-3
Lamkanfi M, Dixit VM (2012) Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol 28:137–161. https://doi.org/10.1146/annurev-cellbio-101011-155745
Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40(2):140–155. https://doi.org/10.1002/glia.10161
Freeman L, Guo H, David CN, Brickey WJ, Jha S, Ting JP (2017) NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med 214(5):1351–1370. https://doi.org/10.1084/jem.20150237
Theriault P, ElAli A, Rivest S (2015) The dynamics of monocytes and microglia in Alzheimer’s disease. Alzheimers Res Ther 7(1):41. https://doi.org/10.1186/s13195-015-0125-2
Ou Z, Zhou Y, Wang L, Xue L, Zheng J, Chen L, Tong Q (2021) NLRP3 inflammasome inhibition prevents alpha-synuclein pathology by relieving autophagy dysfunction in chronic MPTP-treated NLRP3 knockout mice. Mol Neurobiol 58(4):1303–1311. https://doi.org/10.1007/s12035-020-02198-5
Han C, Yang Y, Guan Q, Zhang X, Shen H, Sheng Y, Wang J, Zhou X, Li W, Guo L, Jiao Q (2020) New mechanism of nerve injury in Alzheimer’s disease: beta-amyloid-induced neuronal pyroptosis. J Cell Mol Med 24(14):8078–8090. https://doi.org/10.1111/jcmm.15439
Lindenberg R (1990) Neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol 49(5):535–537. https://doi.org/10.1097/00005072-199009000-00010
Yiannopoulou KG, Papageorgiou SG (2020) Current and future treatments in Alzheimer disease: an update. J Cent Nerv Syst Dis 12:1179573520907397. https://doi.org/10.1177/1179573520907397
Weiner HL, Frenkel D (2006) Immunology and immunotherapy of Alzheimer’s disease. Nat Rev Immunol 6(5):404–416. https://doi.org/10.1038/nri1843
Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ (2002) Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295(5556):851–855. https://doi.org/10.1126/science.1067484
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9(8):857–865. https://doi.org/10.1038/ni.1636
Murphy N, Grehan B, Lynch MA (2014) Glial uptake of amyloid beta induces NLRP3 inflammasome formation via cathepsin-dependent degradation of NLRP10. NeuroMol Med 16(1):205–215. https://doi.org/10.1007/s12017-013-8274-6
Saresella M, La Rosa F, Piancone F, Zoppis M, Marventano I, Calabrese E, Rainone V, Nemni R et al (2016) The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol Neurodegener 11:23. https://doi.org/10.1186/s13024-016-0088-1
Simard AR, Rivest S (2006) Neuroprotective properties of the innate immune system and bone marrow stem cells in Alzheimer’s disease. Mol Psychiatry 11(4):327–335. https://doi.org/10.1038/sj.mp.4001809
Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D (1989) Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol 24(3):173–182. https://doi.org/10.1016/0165-5728(89)90115-x
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D et al (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493(7434):674–678. https://doi.org/10.1038/nature11729
Martin E, Amar M, Dalle C, Youssef I, Boucher C, Le Duigou C, Bruckner M, Prigent A et al (2019) New role of P2X7 receptor in an Alzheimer’s disease mouse model. Mol Psychiatry 24(1):108–125. https://doi.org/10.1038/s41380-018-0108-3
Gemma C, Bickford PC (2007) Interleukin-1beta and caspase-1: players in the regulation of age-related cognitive dysfunction. Rev Neurosci 18(2):137–148. https://doi.org/10.1515/revneuro.2007.18.2.137
Bourgognon JM, Cavanagh J (2020) The role of cytokines in modulating learning and memory and brain plasticity. Brain Neurosci Adv 4:2398212820979802. https://doi.org/10.1177/2398212820979802
Wang Z, Liu D, Wang F, Liu S, Zhao S, Ling EA, Hao A (2012) Saturated fatty acids activate microglia via Toll-like receptor 4/NF-kappaB signalling. Br J Nutr 107(2):229–241. https://doi.org/10.1017/S0007114511002868
Gupta S, Knight AG, Gupta S, Keller JN, Bruce-Keller AJ (2012) Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J Neurochem 120(6):1060–1071. https://doi.org/10.1111/j.1471-4159.2012.07660.x
Liu L, Chan C (2014) IPAF inflammasome is involved in interleukin-1beta production from astrocytes, induced by palmitate; implications for Alzheimer’s Disease. Neurobiol Aging 35(2):309–321. https://doi.org/10.1016/j.neurobiolaging.2013.08.016
Gcwensa NZ, Russell DL, Cowell RM, Volpicelli-Daley LA (2021) Molecular mechanisms underlying synaptic and axon degeneration in Parkinson’s disease. Front Cell Neurosci 15:626128. https://doi.org/10.3389/fncel.2021.626128
Burke RE, O’Malley K (2013) Axon degeneration in Parkinson’s disease. Exp Neurol 246:72–83. https://doi.org/10.1016/j.expneurol.2012.01.011
Anderson FL, Coffey MM, Berwin BL, Havrda MC (2018) Inflammasomes: an emerging mechanism translating environmental toxicant exposure into neuroinflammation in Parkinson’s disease. Toxicol Sci 166(1):3–15. https://doi.org/10.1093/toxsci/kfy219
Voet S, Srinivasan S, Lamkanfi M, van Loo G (2019) Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol Med 11(6):e10248. https://doi.org/10.15252/emmm.201810248
Whitton PS (2007) Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol 150(8):963–976. https://doi.org/10.1038/sj.bjp.0707167
Drouin-Ouellet J, St-Amour I, Saint-Pierre M, Lamontagne-Proulx J, Kriz J, Barker RA, Cicchetti F (2014) Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson's disease. Int J Neuropsychopharmacol 18(6):pyu103. https://doi.org/10.1093/ijnp/pyu103
Nayak D, Roth TL, McGavern DB (2014) Microglia development and function. Annu Rev Immunol 32:367–402. https://doi.org/10.1146/annurev-immunol-032713-120240
Litteljohn D, Cummings A, Brennan A, Gill A, Chunduri S, Anisman H, Hayley S (2010) Interferon-gamma deficiency modifies the effects of a chronic stressor in mice: implications for psychological pathology. Brain Behav Immun 24(3):462–473. https://doi.org/10.1016/j.bbi.2009.12.001
Knott C, Stern G, Wilkin GP (2000) Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci 16(6):724–739. https://doi.org/10.1006/mcne.2000.0914
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38(8):1285–1291. https://doi.org/10.1212/wnl.38.8.1285
Lawana V, Singh N, Sarkar S, Charli A, Jin H, Anantharam V, Kanthasamy AG, Kanthasamy A (2017) Involvement of c-Abl kinase in microglial activation of NLRP3 inflammasome and impairment in autolysosomal system. J Neuroimmune Pharmacol 12(4):624–660. https://doi.org/10.1007/s11481-017-9746-5
Zhang P, Shao XY, Qi GJ, Chen Q, Bu LL, Chen LJ, Shi J, Ming J et al (2016) Cdk5-dependent activation of neuronal inflammasomes in Parkinson’s disease. Mov Disord 31(3):366–376. https://doi.org/10.1002/mds.26488
Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, de Bernard M (2013) Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS ONE 8(1):e55375. https://doi.org/10.1371/journal.pone.0055375
Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P (1995) Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202(1–2):17–20. https://doi.org/10.1016/0304-3940(95)12192-7
Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y (2016) Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol 73(11):1316–1324. https://doi.org/10.1001/jamaneurol.2016.2742
Fan Z, Pan YT, Zhang ZY, Yang H, Yu SY, Zheng Y, Ma JH, Wang XM (2020) Systemic activation of NLRP3 inflammasome and plasma alpha-synuclein levels are correlated with motor severity and progression in Parkinson’s disease. J Neuroinflammation 17(1):11. https://doi.org/10.1186/s12974-019-1670-6
Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T (1994) Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett 180(2):147–150. https://doi.org/10.1016/0304-3940(94)90508-8
Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R (2015) Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160(1–2):62–73. https://doi.org/10.1016/j.cell.2014.11.047
Nopoulos PC (2016) Huntington disease: a single-gene degenerative disorder of the striatum. Dialogues Clin Neurosci 18(1):91–98
Roos RA (2010) Huntington’s disease: a clinical review. Orphanet J Rare Dis 5:40. https://doi.org/10.1186/1750-1172-5-40
Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE et al (1997) Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90(3):537–548. https://doi.org/10.1016/s0092-8674(00)80513-9
Sanchez Mejia RO, Friedlander RM (2001) Caspases in Huntington’s disease. Neuroscientist 7(6):480–489. https://doi.org/10.1177/107385840100700604
Ona VO, Li M, Vonsattel JP, Andrews LJ, Khan SQ, Chung WM, Frey AS, Menon AS et al (1999) Inhibition of caspase-1 slows disease progression in a mouse model of Huntington’s disease. Nature 399(6733):263–267. https://doi.org/10.1038/20446
Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L et al (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6(7):797–801. https://doi.org/10.1038/77528
Compston A (2004) The pathogenesis and basis for treatment in multiple sclerosis. Clin Neurol Neurosurg 106(3):246–248. https://doi.org/10.1016/j.clineuro.2004.02.007
Goverman J (2009) Autoimmune T cell responses in the central nervous system. Nat Rev Immunol 9(6):393–407. https://doi.org/10.1038/nri2550
Andersson A, Covacu R, Sunnemark D, Danilov AI, Dal Bianco A, Khademi M, Wallstrom E, Lobell A et al (2008) Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J Leukoc Biol 84(5):1248–1255. https://doi.org/10.1189/jlb.1207844
Malhotra S, Costa C, Eixarch H, Keller CW, Amman L, Martinez-Banaclocha H, Midaglia L, Sarro E et al (2020) NLRP3 inflammasome as prognostic factor and therapeutic target in primary progressive multiple sclerosis patients. Brain 143(5):1414–1430. https://doi.org/10.1093/brain/awaa084
Inoue M, Shinohara ML (2013) NLRP3 Inflammasome and MS/EAE. Autoimmune Dis 2013:859145. https://doi.org/10.1155/2013/859145
Mamik MK, Power C (2017) Inflammasomes in neurological diseases: emerging pathogenic and therapeutic concepts. Brain 140(9):2273–2285. https://doi.org/10.1093/brain/awx133
Russi AE, Walker-Caulfield ME, Guo Y, Lucchinetti CF, Brown MA (2016) Meningeal mast cell-T cell crosstalk regulates T cell encephalitogenicity. J Autoimmun 73:100–110. https://doi.org/10.1016/j.jaut.2016.06.015
Voet S, Mc Guire C, Hagemeyer N, Martens A, Schroeder A, Wieghofer P, Daems C, Staszewski O et al (2018) A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat Commun 9(1):2036. https://doi.org/10.1038/s41467-018-04376-5
Keane RW, Dietrich WD, de Rivero Vaccari JP (2018) Inflammasome proteins as biomarkers of multiple sclerosis. Front Neurol 9:135. https://doi.org/10.3389/fneur.2018.00135
Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS et al (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21(3):248–255. https://doi.org/10.1038/nm.3806
Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L (2007) Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol 8(1):74–83. https://doi.org/10.1038/ni1415
Prusiner SB, Hsiao KK (1994) Human prion diseases. Ann Neurol 35(4):385–395. https://doi.org/10.1002/ana.410350404
Van Everbroeck B, Dobbeleir I, De Waele M, De Deyn P, Martin JJ, Cras P (2004) Differential diagnosis of 201 possible Creutzfeldt-Jakob disease patients. J Neurol 251(3):298–304. https://doi.org/10.1007/s00415-004-0311-9
Zhu C, Herrmann US, Falsig J, Abakumova I, Nuvolone M, Schwarz P, Frauenknecht K, Rushing EJ et al (2016) A neuroprotective role for microglia in prion diseases. J Exp Med 213(6):1047–1059. https://doi.org/10.1084/jem.20151000
Shi F, Yang L, Kouadir M, Yang Y, Wang J, Zhou X, Yin X, Zhao D (2012) The NALP3 inflammasome is involved in neurotoxic prion peptide-induced microglial activation. J Neuroinflammation 9:73. https://doi.org/10.1186/1742-2094-9-73
Sasaki A, Hirato J, Nakazato Y (1993) Immunohistochemical study of microglia in the Creutzfeldt-Jakob diseased brain. Acta Neuropathol 86(4):337–344. https://doi.org/10.1007/BF00369445
Giese A, Brown DR, Groschup MH, Feldmann C, Haist I, Kretzschmar HA (1998) Role of microglia in neuronal cell death in prion disease. Brain Pathol 8(3):449–457. https://doi.org/10.1111/j.1750-3639.1998.tb00167.x
Hafner-Bratkovic I, Bencina M, Fitzgerald KA, Golenbock D, Jerala R (2012) NLRP3 inflammasome activation in macrophage cell lines by prion protein fibrils as the source of IL-1beta and neuronal toxicity. Cell Mol Life Sci 69(24):4215–4228. https://doi.org/10.1007/s00018-012-1140-0
Srivastava S, Katorcha E, Makarava N, Barrett JP, Loane DJ, Baskakov IV (2018) Inflammatory response of microglia to prions is controlled by sialylation of PrP(Sc). Sci Rep 8(1):11326. https://doi.org/10.1038/s41598-018-29720-z
Bellezza I, Grottelli S, Costanzi E, Scarpelli P, Pigna E, Morozzi G, Mezzasoma L, Peirce MJ et al (2018) Peroxynitrite activates the NLRP3 inflammasome cascade in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Mol Neurobiol 55(3):2350–2361. https://doi.org/10.1007/s12035-017-0502-x
Borchelt DR, Lee MK, Slunt HS, Guarnieri M, Xu ZS, Wong PC, Brown RH Jr, Price DL et al (1994) Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci U S A 91(17):8292–8296. https://doi.org/10.1073/pnas.91.17.8292
Gugliandolo A, Giacoppo S, Bramanti P, Mazzon E (2018) NLRP3 inflammasome activation in a transgenic amyotrophic lateral sclerosis model. Inflammation 41(1):93–103. https://doi.org/10.1007/s10753-017-0667-5
Pasinelli P, Borchelt DR, Houseweart MK, Cleveland DW, Brown RH Jr (1998) Caspase-1 is activated in neural cells and tissue with amyotrophic lateral sclerosis-associated mutations in copper-zinc superoxide dismutase. Proc Natl Acad Sci U S A 95(26):15763–15768. https://doi.org/10.1073/pnas.95.26.15763
Johann S, Heitzer M, Kanagaratnam M, Goswami A, Rizo T, Weis J, Troost D, Beyer C (2015) NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia 63(12):2260–2273. https://doi.org/10.1002/glia.22891
Meissner F, Molawi K, Zychlinsky A (2010) Mutant superoxide dismutase 1-induced IL-1beta accelerates ALS pathogenesis. Proc Natl Acad Sci U S A 107(29):13046–13050. https://doi.org/10.1073/pnas.1002396107
Lehmann S, Esch E, Hartmann P, Goswami A, Nikolin S, Weis J, Beyer C, Johann S (2018) Expression profile of pattern recognition receptors in skeletal muscle of SOD1((G93A)) amyotrophic lateral sclerosis (ALS) mice and sporadic ALS patients. Neuropathol Appl Neurobiol 44(6):606–627. https://doi.org/10.1111/nan.12483
De Paola M, Sestito SE, Mariani A, Memo C, Fanelli R, Freschi M, Bendotti C, Calabrese V et al (2016) Synthetic and natural small molecule TLR4 antagonists inhibit motoneuron death in cultures from ALS mouse model. Pharmacol Res 103:180–187. https://doi.org/10.1016/j.phrs.2015.11.020
Beers DR, Henkel JS, Zhao W, Wang J, Appel SH (2008) CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci U S A 105(40):15558–15563. https://doi.org/10.1073/pnas.0807419105
Henkel JS, Beers DR, Zhao W, Appel SH (2009) Microglia in ALS: the good, the bad, and the resting. J Neuroimmune Pharmacol 4(4):389–398. https://doi.org/10.1007/s11481-009-9171-5
Rothstein JD, Tsai G, Kuncl RW, Clawson L, Cornblath DR, Drachman DB, Pestronk A, Stauch BL et al (1990) Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol 28(1):18–25. https://doi.org/10.1002/ana.410280106
Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li P, Chen FF, Jiang XD (2013) Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation 10:106. https://doi.org/10.1186/1742-2094-10-106
Kerr N, Lee SW, Perez-Barcena J, Crespi C, Ibanez J, Bullock MR, Dietrich WD, Keane RW et al (2018) Inflammasome proteins as biomarkers of traumatic brain injury. PLoS ONE 13(12):e0210128. https://doi.org/10.1371/journal.pone.0210128
Irrera N, Pizzino G, Calo M, Pallio G, Mannino F, Fama F, Arcoraci V, Fodale V et al (2017) Lack of the Nlrp3 inflammasome improves mice recovery following traumatic brain injury. Front Pharmacol 8:459. https://doi.org/10.3389/fphar.2017.00459
Weber JT (2012) Altered calcium signaling following traumatic brain injury. Front Pharmacol 3:60. https://doi.org/10.3389/fphar.2012.00060
Awasthi D, Kutz SC, Beuerman R, Nguyen D, Carey ME, Zeiller S (2003) Early gene expression in the rat cortex after experimental traumatic brain injury and hypotension. Neurosci Lett 345(1):29–32. https://doi.org/10.1016/s0304-3940(03)00497-x
Clausen F, Hanell A, Bjork M, Hillered L, Mir AK, Gram H, Marklund N (2009) Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 30(3):385–396. https://doi.org/10.1111/j.1460-9568.2009.06820.x
Adamczak S, Dale G, de Rivero Vaccari JP, Bullock MR, Dietrich WD, Keane RW (2012) Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg 117(6):1119–1125. https://doi.org/10.3171/2012.9.JNS12815
Wallisch JS, Simon DW, Bayir H, Bell MJ, Kochanek PM, Clark RSB (2017) Cerebrospinal fluid NLRP3 is increased after severe traumatic brain injury in infants and children. Neurocrit Care 27(1):44–50. https://doi.org/10.1007/s12028-017-0378-7
Piancone F, La Rosa F, Marventano I, Saresella M, Clerici M (2021) The role of the inflammasome in neurodegenerative diseases. Molecules 26(4):953. https://doi.org/10.3390/molecules26040953
Duan Y, Kelley N, He Y (2020) Role of the NLRP3 inflammasome in neurodegenerative diseases and therapeutic implications. Neural Regen Res 15(7):1249–1250. https://doi.org/10.4103/1673-5374.272576
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N et al (2015) The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21(3):263–269. https://doi.org/10.1038/nm.3804
Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, Meng R, Quong AA et al (2010) Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct inhibitors of the inflammasome. J Biol Chem 285(13):9792–9802. https://doi.org/10.1074/jbc.M109.082305
Ahn H, Kim J, Jeung EB, Lee GS (2014) Dimethyl sulfoxide inhibits NLRP3 inflammasome activation. Immunobiology 219(4):315–322. https://doi.org/10.1016/j.imbio.2013.11.003
Inoue M, Williams KL, Oliver T, Vandenabeele P, Rajan JV, Miao EA, Shinohara ML (2012) Interferon-beta therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci Signal 5(225):ra38. https://doi.org/10.1126/scisignal.2002767
Isakov E, Weisman-Shomer P (1840) Benhar M (2014) Suppression of the pro-inflammatory NLRP3/interleukin-1beta pathway in macrophages by the thioredoxin reductase inhibitor auranofin. Biochim Biophys Acta 10:3153–3161. https://doi.org/10.1016/j.bbagen.2014.07.012
Honda H, Nagai Y, Matsunaga T, Okamoto N, Watanabe Y, Tsuneyama K, Hayashi H, Fujii I et al (2014) Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J Leukoc Biol 96(6):1087–1100. https://doi.org/10.1189/jlb.3A0114-005RR
He Y, Varadarajan S, Munoz-Planillo R, Burberry A, Nakamura Y, Nunez G (2014) 3,4-methylenedioxy-beta-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem 289(2):1142–1150. https://doi.org/10.1074/jbc.M113.515080
Maier NK, Leppla SH, Moayeri M (2015) The cyclopentenone prostaglandin 15d-PGJ2 inhibits the NLRP1 and NLRP3 inflammasomes. J Immunol 194(6):2776–2785. https://doi.org/10.4049/jimmunol.1401611
Reboldi A, Dang EV, McDonald JG, Liang G, Russell DW, Cyster JG (2014) Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 345(6197):679–684. https://doi.org/10.1126/science.1254790
Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, Robertson AAB, Butler MS, Rowe DB, O'Neill LA, Kanthasamy AG, Schroder K, Cooper MA, Woodruff TM (2018) Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med 10(465):eaah4066. https://doi.org/10.1126/scitranslmed.aah4066
Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, Robertson AAB, Cooper MA, O’Neill LAJ, Lynch MA (2017) Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav Immun 61:306–316. https://doi.org/10.1016/j.bbi.2016.12.014
La Rosa F, Saresella M, Marventano I, Piancone F, Ripamonti E, Al-Daghri N, Bazzini C, Zoia CP, Conti E, Ferrarese C, Clerici M (2019) Stavudine reduces NLRP3 inflammasome activation and modulates amyloid-beta autophagy. J Alzheimers Dis 72(2):401–412. https://doi.org/10.3233/JAD-181259
Acknowledgements
The authors gratefully acknowledge the Department of Science and Technology, Government of India, for their support in the form of FIST Grant.
Funding
This work was supported by the Science & Engineering Research Board, Government of India (EMR/2017/002793); the University Grants Commission, Government of India (Startup Research Grant); and the Indian Council of Medical Research, Government of India (IRIS ID: 2020–5687).
Author information
Authors and Affiliations
Contributions
LC outlined and structured the review; SB, NS and LC wrote the manuscript; NS and LC created the figures and tables; SB, NS, and LC proofed the text and edited the tables and figures. LC and NS revised the manuscript. All authors read and approve the final version of the manuscript at the time of submission.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All contributing authors agree to the publication of this article.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brahadeeswaran, S., Sivagurunathan, N. & Calivarathan, L. Inflammasome Signaling in the Aging Brain and Age-Related Neurodegenerative Diseases. Mol Neurobiol 59, 2288–2304 (2022). https://doi.org/10.1007/s12035-021-02683-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02683-5