Abstract
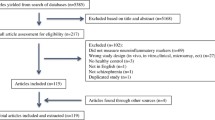
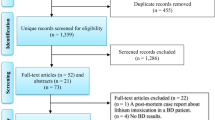
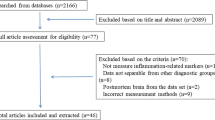
There have been a large number of reports about glial cell dysfunction being related to major psychiatric diseases such as schizophrenia (SCZ), bipolar disorder (BD), and major depressive disorder (MDD). In this review, we provide an overview of postmortem studies analyzing the structural changes of glial cells in these three major psychiatric diseases, including the density, number and size of glial cells, and the expression of related markers. Up to May 1, 2021, 108 articles that met the inclusion criteria were identified by searching PubMed and Web of Science. Although most studies evaluating total glial cells did not show abnormalities in the brains of postmortem patients, astrocytes, microglial cells, and oligodendrocytes seem to have specific patterns of changes in each disease. For example, out of 20 studies that evaluated astrocyte markers in MDD, 11 studies found decreased astrocyte marker expression in MDD patients. Similarly, out of 25 studies evaluating oligodendrocyte markers in SCZ, 15 studies showed decreased expression of oligodendrocyte markers in different brain regions of SCZ patients. In addition, activated microglial cells were observed in patients with SCZ, BD, and MDD, but suicide may be a confounding factor for the observed effects. Although the data from the included studies were heterogeneous and this cannot be fully explained at present, it is likely that there are a variety of contributing factors, including the measured brain regions, methods of measurement, gender, age at time of death, and medications.



Similar content being viewed by others
Data Availability
All data are contained within the manuscript.
References
Somjen G (1988) Nervenkitt: notes on the history of the concept of neuroglia. Glia 1:2–9
Miller, G. Neuroscience. The dark side of glia. Science (New York, N.Y.) 308, 778–781 (2005).
Cotter D, Pariante C, Everall I (2001) Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull 55:585–595
Barres B (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60:430–440
Azevedo FA, Carvalho LR et al (2009) Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513:532–541
Otte C, Gold S et al (2016) Major depressive disorder. Nat Rev Dis Primers 2:16065
Kahn R, Sommer I et al (2015) Schizophrenia. Nat Rev Dis Primers 1:15067
Carvalho A, Firth J, Vieta E (2020) Bipolar disorder. N Engl J Med 383:58–66
Goldsmith D, Rapaport M, Miller B (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709
Enache D, Pariante C, Mondelli V (2019) Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun 81:24–40
Giridharan V, Sayana P et al (2020) Postmortem evidence of brain inflammatory markers in bipolar disorder: a systematic review. Mol Psychiatry 25:94–113
Trépanier M, Hopperton K, Mizrahi R, Mechawar N, Bazinet R (2016) Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry 21:1009–1026
Sild M, Ruthazer E, Booij L (2017) Major depressive disorder and anxiety disorders from the glial perspective: etiological mechanisms, intervention and monitoring. Neurosci Biobehav Rev 83:474–488
Gigase F, Snijders G, Boks M, de Witte L (2019) Neurons and glial cells in bipolar disorder: a systematic review of postmortem brain studies of cell number and size. Neurosci Biobehav Rev 103:150–162
Dietz A, Goldman S, Nedergaard M (2020) Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry 7:272–281
Altshuler L, Abulseoud O et al (2010) Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord 12:541–549
Barley K, Dracheva S, Byne W (2009) Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res 112:54–64
Beasley C, Honavar M, Everall I, Cotter D (2009) Two-dimensional assessment of cytoarchitecture in the superior temporal white matter in schizophrenia, major depressive disorder and bipolar disorder. Schizophr Res 115:156–162
Benes F, Davidson J, Bird E (1986) Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry 43:31–35
Benes F, Vincent S, Todtenkopf M (2001) The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiat 50:395–406
Bernard R, Kerman I et al (2011) Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 16:634–646
Bezchlibnyk Y, Sun X et al (2007) Neuron somal size is decreased in the lateral amygdalar nucleus of subjects with bipolar disorder. J Psychiatry Neuroscience : JPN 32:203–210
Boldrini M, Galfalvy H et al (2019) Resilience is associated with larger dentate gyrus, while suicide decedents with major depressive disorder have fewer granule neurons. Biol Psychiatry 85:850–862
Brauch, R., Adnan El-Masri, M., Parker, J. and El-Mallakh, R. Glial cell number and neuron/glial cell ratios in postmortem brains of bipolar individuals. Journal of affective disorders 91, 87–90 (2006).
Brisch R, Steiner J et al (2017) Microglia in the dorsal raphe nucleus plays a potential role in both suicide facilitation and prevention in affective disorders. Eur Arch Psychiatry Clin Neurosci 267:403–415
Busse S, Busse M et al (2012) Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun 26:1273–1279
Byne W, Kidkardnee S et al (2006) Schizophrenia-associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophr Res 85:245–253
Association with neuroinflammation (2014) Catts, V.S., Wong, J., Fillman, S.G., Fung, S.J. and Shannon Weickert, C. Increased expression of astrocyte markers in schizophrenia. Aust N Z J Psychiatry 48:722–734
Benes, F., McSparren, J., Bird, E., San Giovanni, J. and Vincent, S. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 48, 996–1001 (1991).
Chana G, Landau S, Beasley C, Everall IP, Cotter D (2003) Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry 53:1086–1098
Byne W, Dracheva S et al (2008) Schizophrenia and sex associated differences in the expression of neuronal and oligodendrocyte-specific genes in individual thalamic nuclei. Schizophr Res 98:118–128
Clark SM, Pocivavsek A et al (2016) Reduced kynurenine pathway metabolism and cytokine expression in the prefrontal cortex of depressed individuals. J Psychiatry Neurosci 41:386–394
Cobb J, O’Neill K et al (2016) Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience 316:209–220
Cotter D, Hudson L, Landau S (2005) Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord 7:358–369
Cotter, D., Mackay, D. et al. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex (New York, N.Y. : 1991) 12, 386–394 (2002).
Cotter D, Mackay D, Frangou S, Hudson L, Landau S (2004) Cell density and cortical thickness in Heschl’s gyrus in schizophrenia, major depression and bipolar disorder. British J Psychiatry : J Ment Sci 185:258–259
Cotter D, Mackay D, Landau S, Kerwin R, Everall I (2001) Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 58:545–553
Davis S, Thomas A et al (2002) Glial fibrillary acidic protein in late life major depressive disorder: an immunocytochemical study. J Neurol Neurosurg Psychiatry 73:556–560
Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S (2010) Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry 67:155–166
Durrenberger, P., Fernando, F. et al. Common mechanisms in neurodegeneration and neuroinflammation: a BrainNet Europe gene expression microarray study. J Neural Transm (Vienna, Austria : 1996) 122, 1055–1068 (2015).
Falkai P, Bogerts B (1986) Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 236:154–161
Falkai P, Bogerts B, Rozumek M (1988) Limbic pathology in schizophrenia: the entorhinal region–a morphometric study. Biol Psychiat 24:515–521
Radewicz K, Garey LJ, Gentleman SM, Reynolds R (2000) Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol 59:137–150
Falkai, P., Malchow, B. et al. Decreased oligodendrocyte and neuron number in anterior hippocampal areas and the entire hippocampus in schizophrenia: a stereological postmortem study. Schizophr Bull S4-S12 (2016).
Farkas N, Lendeckel U et al (2010) Reduced density of ADAM 12-immunoreactive oligodendrocytes in the anterior cingulate white matter of patients with schizophrenia. World J Biol Psychiatry : Off J World Fed Soc Biol Psychiatry 11:556–566
Fatemi S, Laurence J et al (2004) Glial fibrillary acidic protein is reduced in cerebellum of subjects with major depression, but not schizophrenia. Schizophr Res 69:317–323
Feresten A, Barakauskas V, Ypsilanti A, Barr A, Beasley C (2013) Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness. Schizophr Res 150:252–257
Gos T, Schroeter M et al (2013) S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: a postmortem study. J Psychiatr Res 47:1694–1699
Gos T, Myint AM et al (2014) Reduced microglial immunoreactivity for endogenous NMDA receptor agonist quinolinic acid in the hippocampus of schizophrenia patients. Brain Behav Immun 41:59–64
Hamidi M, Drevets W, Price J (2004) Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiat 55:563–569
Hercher C, Chopra V, Beasley CL (2014) Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J Psychiatry Neurosci 39:376–385
Hof P, Haroutunian V et al (2003) Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiat 53:1075–1085
Höistad M, Heinsen H, Wicinski B, Schmitz C, Hof P (2013) Stereological assessment of the dorsal anterior cingulate cortex in schizophrenia: absence of changes in neuronal and glial densities. Neuropathol Appl Neurobiol 39:348–361
Karson C, Casanova M, Kleinman J, Griffin W (1993) Choline acetyltransferase in schizophrenia. Am J Psychiatry 150:454–459
Kerns D, Vong G et al (2010) Gene expression abnormalities and oligodendrocyte deficits in the internal capsule in schizophrenia. Schizophr Res 120:150–158
Khundakar A, Morris C, Oakley A, McMeekin W, Thomas A (2009) Morphometric analysis of neuronal and glial cell pathology in the dorsolateral prefrontal cortex in late-life depression. The British journal of psychiatry : the journal of mental science 195:163–169
Khundakar A, Morris C, Oakley A, Thomas A (2011) A morphometric examination of neuronal and glial cell pathology in the orbitofrontal cortex in late-life depression. Int Psychogeriatr 23:132–140
Khundakar A, Morris C, Oakley A, Thomas A (2011) Morphometric analysis of neuronal and glial cell pathology in the caudate nucleus in late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 19:132–141
Khundakar A, Morris C, Oakley A, Thomas A (2011) Cellular pathology within the anterior cingulate cortex of patients with late-life depression: a morphometric study. Psychiatry Res 194:184–189
Kolomeets N, Uranova N (2019) Reduced oligodendrocyte density in layer 5 of the prefrontal cortex in schizophrenia. Eur Arch Psychiatry Clin Neurosci 269:379–386
Kolomeets NS, Uranova NA (2020) Numerical density of oligodendrocytes and oligodendrocyte clusters in the anterior putamen in major psychiatric disorders. Eur Arch Psychiatry Clin Neurosci 270:841–850
Lopez-Gonzalez I, Pinacho R et al (2019) Neuroinflammation in the dorsolateral prefrontal cortex in elderly chronic schizophrenia. Eur Neuropsychopharmacol 29:384–396
Martins-de-Souza D, Gattaz W et al (2009) Proteomic analysis of dorsolateral prefrontal cortex indicates the involvement of cytoskeleton, oligodendrocyte, energy metabolism and new potential markers in schizophrenia. J Psychiatr Res 43:978–986
Mauney S, Pietersen C, Sonntag K, Woo T (2015) Differentiation of oligodendrocyte precursors is impaired in the prefrontal cortex in schizophrenia. Schizophr Res 169:374–380
Miguel-Hidalgo J, Baucom C et al (2000) Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiat 48:861–873
Miguel-Hidalgo J, Waltzer R et al (2010) Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord 127:230–240
Nagy C, Suderman M et al (2015) Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry 20:320–328
Nasrallah H, McCalley-Whitters M, Bigelow L, Rauscher F (1983) A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res 8:251–260
Nishioka N, Arnold SE (2004) Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry 12:167–175
O'Leary, L.A., Belliveau, C. et al. Widespread decrease of cerebral vimentin-immunoreactive astrocytes in depressed suicides. Front Psychiatry 12, 640963 (2021).
Petrasch-Parwez, E., Schöbel, A. et al. Lateralization of increased density of Iba1-immunopositive microglial cells in the anterior midcingulate cortex of schizophrenia and bipolar disorder. European archives of psychiatry and clinical neuroscience (2020).
Qi XR, Kamphuis W, Shan L (2019) Astrocyte changes in the prefrontal cortex from aged non-suicidal depressed patients. Front Cell Neurosci 13:503
Rajkowska G, Halaris A, Selemon L (2001) Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiat 49:741–752
Rajkowska G, Miguel-Hidalgo JJ et al (2002) Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res 57:127–138
Rajkowska G, Miguel-Hidalgo J, Dubey P, Stockmeier C, Krishnan K (2005) Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiat 58:297–306
Rajkowska G, Mahajan G et al (2015) Oligodendrocyte morphometry and expression of myelin - Related mRNA in ventral prefrontal white matter in major depressive disorder. J Psychiatr Res 65:53–62
Rao J, Harry G, Kim H (2010) Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry 15:384–392
Di Rosa E, Crow T, Walker M, Black G, Chance S (2009) Reduced neuron density, enlarged minicolumn spacing and altered ageing effects in fusiform cortex in schizophrenia. Psychiatry Res 166:102–115
Schmitt A, Steyskal C et al (2009) Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol 117:395–407
Schmitt A, Leonardi-Essmann F et al (2011) Regulation of immune-modulatory genes in left superior temporal cortex of schizophrenia patients: a genome-wide microarray study. World J Biol Psychiatry 12:201–215
Segal D, Schmitz C, Hof P (2009) Spatial distribution and density of oligodendrocytes in the cingulum bundle are unaltered in schizophrenia. Acta Neuropathol 117:385–394
Selemon L, Mrzljak J, Kleinman J, Herman M, Goldman-Rakic P (2003) Regional specificity in the neuropathologic substrates of schizophrenia: a morphometric analysis of Broca’s area 44 and area 9. Arch Gen Psychiatry 60:69–77
Selemon L, Begovic A (2007) Stereologic analysis of the lateral geniculate nucleus of the thalamus in normal and schizophrenic subjects. Psychiatry Res 151:1–10
Seredenina T, Sorce S et al (2017) Decreased NOX2 expression in the brain of patients with bipolar disorder: association with valproic acid prescription and substance abuse. Transl Psychiatry 7:e1206
Shimamoto-Mitsuyama C, Nakaya A et al (2021) Lipid pathology of the corpus callosum in schizophrenia and the potential role of abnormal gene regulatory networks with reduced microglial marker expression. Cereb Cortex 31:448–462
Si X, Miguel-Hidalgo J, O’Dwyer G, Stockmeier C, Rajkowska G (2004) Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 29:2088–2096
Smiley J, Hackett T et al (2016) Reduced GABA neuron density in auditory cerebral cortex of subjects with major depressive disorder. J Chem Neuroanat 76:108–121
Snijders G, van Zuiden W et al (2021) A loss of mature microglial markers without immune activation in schizophrenia. Glia 69:1251–1267
Steiner J, Bielau H et al (2008) Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res 42:151–157
Steiner J, Mawrin C et al (2006) Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol 112:305–316
Stockmeier C, Mahajan G et al (2004) Cellular changes in the postmortem hippocampus in major depression. Biol Psychiat 56:640–650
Tanti A, Kim J et al (2018) Child abuse associates with an imbalance of oligodendrocyte-lineage cells in ventromedial prefrontal white matter. Mol Psychiatry 23:2018–2028
Tkachev D, Mimmack ML et al (2003) Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 362:798–805
Torii, Y., Iritani, S. et al. Morphological alteration of myelin-oligodendrocytes in a schizophrenic patient with 22q11.2 deletion syndrome Schizophr Res 223:353–355
Torres-Platas S, Cruceanu C, Chen G, Turecki G, Mechawar N (2014) Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 42:50–59
Torres-Platas S, Hercher C et al (2011) Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 36:2650–2658
Torres-Platas SG, Nagy C, Wakid M, Turecki G, Mechawar N (2016) Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol Psychiatry 21:509–515
Tzioras M, Stevenson AJ, Boche D, Spires-Jones TL (2021) Microglial contribution to synaptic uptake in the prefrontal cortex in schizophrenia. Neuropathol Appl Neurobiol 47:346–351
Uranova N, Orlovskaya D et al (2001) Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull 55:597–610
Uranova N, Vikhreva O, Rakhmanova V, Orlovskaya D (2018) Ultrastructural pathology of oligodendrocytes adjacent to microglia in prefrontal white matter in schizophrenia. NPJ Schizophr 4:26
Uranova N, Vikhreva O, Rakhmanova V, Orlovskaya D (2020) Dystrophy of oligodendrocytes and adjacent microglia in prefrontal gray matter in schizophrenia. Front Psych 11:204
Uranova N, Vostrikov V, Orlovskaya D, Rachmanova V (2004) Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res 67:269–275
Vikhreva O, Rakhmanova V, Orlovskaya D, Uranova N (2016) Ultrastructural alterations of oligodendrocytes in prefrontal white matter in schizophrenia: A post-mortem morphometric study. Schizophr Res 177:28–36
Vostrikov V, Orlovskaya D, Uranova N (2008) Deficit of pericapillary oligodendrocytes in the prefrontal cortex in schizophrenia. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry 9:34–42
Walker C, Roche J, Sinha V, Roberts R (2018) Substantia nigra ultrastructural pathology in schizophrenia. Schizophr Res 197:209–218
Webster MJ, O’Grady J, Kleinman JE, Weickert CS (2005) Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience 133:453–461
Wesseling, H., Gottschalk, M. and Bahn, S. Targeted multiplexed selected reaction monitoring analysis evaluates protein expression changes of molecular risk factors for major psychiatric disorders. The international journal of neuropsychopharmacology 18(2014).
Williams MR, Galvin K et al (2014) Neuropathological changes in the substantia nigra in schizophrenia but not depression. Eur Arch Psychiatry Clin Neurosci 264:285–296
Zhang L, Verwer RWH, Lucassen PJ, Huitinga I, Swaab DF (2020) Sex difference in glia gene expression in the dorsolateral prefrontal cortex in bipolar disorder: Relation to psychotic features. J Psychiatr Res 125:66–74
Farnsworth B, Radomska KJ et al (2017) QKI6B mRNA levels are upregulated in schizophrenia and predict GFAP expression. Brain Res 1669:63–68
Marui T, Torii Y et al (2018) The neuropathological study of myelin oligodendrocyte glycoprotein in the temporal lobe of schizophrenia patients. Acta neuropsychiatrica 30:232–240
Müller M, Lucassen P et al (2001) Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci 14:1603–1612
Rajkowska G, Selemon L, Goldman-Rakic P (1998) Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry 55:215–224
Rubinow M, Mahajan G et al (2016) Basolateral amygdala volume and cell numbers in major depressive disorder: a postmortem stereological study. Brain Struct Funct 221:171–184
Sneeboer MAM, van der Doef T et al (2020) Microglial activation in schizophrenia: is translocator 18kDa protein (TSPO) the right marker? Schizophr Res 215:167–172
Sneeboer M, Snijders G et al (2019) Microglia in post-mortem brain tissue of patients with bipolar disorder are not immune activated. Transl Psychiatry 9:153
Cullen T, Walker M et al (2006) Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. The British journal of psychiatry : the journal of mental science 188:26–31
Rajkowska G, Stockmeier C (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14:1225–1236
Chandley M, Szebeni K et al (2013) Gene expression deficits in pontine locus coeruleus astrocytes in men with major depressive disorder. Journal of psychiatry & neuroscience : JPN 38:276–284
Falkai P, Honer WG et al (1999) No evidence for astrogliosis in brains of schizophrenic patients. A post-mortem study Neuropathol Appl Neurobiol 25:48–53
Damadzic R, Bigelow LB et al (2001) A quantitative immunohistochemical study of astrocytes in the entorhinal cortex in schizophrenia, bipolar disorder and major depression: absence of significant astrocytosis. Brain Res Bull 55:611–618
Malchow, B., Strocka, S. et al. Stereological investigation of the posterior hippocampus in affective disorders. Journal of neural transmission (Vienna, Austria : 1996) 122, 1019–1033 (2015).
Hercher C, Chopra V, Beasley C (2014) Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. Journal of psychiatry & neuroscience : JPN 39:376–385
Czeh, B, Di Benedetto, B. Antidepressants act directly on astrocytes Eur Neuropsychopharmacol 23:171–185
Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E (2006) Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology 31:1616–1626
Czeh B, Muller-Keuker JI et al (2007) Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology 32:1490–1503
Kodama M, Fujioka T, Duman RS (2004) Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry 56:570–580
Toro CT, Hallak JE, Dunham JS, Deakin JF (2006) Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neurosci Lett 404:276–281
Hendrickx DAE, van Eden CG, Schuurman KG, Hamann J, Huitinga I (2017) Staining of HLA-DR, Iba1 and CD68 in human microglia reveals partially overlapping expression depending on cellular morphology and pathology. J Neuroimmunol 309:12–22
Pandey GN, Rizavi HS et al (2012) Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res 46:57–63
Voineskos AN, Felsky D et al (2013) Oligodendrocyte genes, white matter tract integrity, and cognition in schizophrenia. Cereb Cortex 23:2044–2057
Takahashi N, Sakurai T, Davis KL, Buxbaum JD (2011) Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol 93:13–24
Fields RD (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31:361–370
Kaufman J, Charney D (2000) Comorbidity of mood and anxiety disorders. Depress Anxiety 12(Suppl 1):69–76
Funding
This study was supported by the National Science Foundation of China (82071676, 81703492).
Author information
Authors and Affiliations
Contributions
All authors have read and agree with the published version of the manuscript. Yong Cheng conceived and designed this study; Shu-Han Liu and Yang Du searched database and identified eligible studies; Shu-Han Liu and Lei Chen extracted the data; all authors critically reviewed the manuscript; Shu-Han Liu drafted the manuscript with critical revisions from Yong Cheng.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, SH., Du, Y., Chen, L. et al. Glial Cell Abnormalities in Major Psychiatric Diseases: A Systematic Review of Postmortem Brain Studies. Mol Neurobiol 59, 1665–1692 (2022). https://doi.org/10.1007/s12035-021-02672-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02672-8