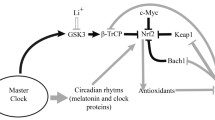
Abstract
Along evolution, living organisms developed a precise timekeeping system, circadian clocks, to adapt life to the 24-h light/dark cycle and temporally regulate physiology and behavior. The transcriptional molecular circadian clock and metabolic/redox oscillator conforming these clocks are present in organs, tissues, and even in individual cells, where they exert circadian control over cellular metabolism. Disruption of the molecular clock may cause metabolic disorders and higher cancer risk. The synthesis and degradation of glycerophospholipids (GPLs) is one of the most highly regulated metabolisms across the 24-h cycle in terms of total lipid content and enzyme expression and activity in the nervous system and individual cells. Lipids play a plethora of roles (membrane biogenesis, energy sourcing, signaling, and the regulation of protein-chromatin interaction, among others), making control of their metabolism a vital checkpoint in the cellular organization of physiology. An increasing body of evidence clearly demonstrates an orchestrated and sequential series of events occurring in GPL metabolism across the 24-h day in diverse retinal cell layers, immortalized fibroblasts, and glioma cells. Moreover, the clock gene Per1 and other circadian-related genes are tightly involved in the regulation of GPL synthesis in quiescent cells. However, under proliferation, the metabolic oscillator continues to control GPL metabolism of brain cancer cells even after molecular circadian clock disruption, reflecting the crucial role of the temporal metabolism organization in cell preservation. The aim of this review is to examine the control exerted by circadian clocks over GPL metabolism, their synthesizing enzyme expression and activities in normal and tumorous cells of the nervous system and in immortalized fibroblasts.
Graphical abstract







Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- AA:
-
Arachidonic acid
- ACC:
-
Acetyl-CoA carboxylase
- AMPK:
-
Adenosine monophosphate-regulated kinase
- BMAL1:
-
Aryl hydrocarbon receptor translocator-like protein 1
- cAMP:
-
Cyclic adenosine monophosphate
- CCG:
-
Clock-controlled genes
- CCT:
-
CTP:phosphocholine cytidylyltransferase
- CET:
-
CTP:ethanolamine cytidylyltransferase
- CG:
-
Clock genes
- ChoK:
-
Choline kinase
- CLOCK:
-
Circadian locomotor output cycles kaput
- CNS:
-
Central nervous system (CNS)
- CPT:
-
CDP‐choline:1,2‐diacylglycerol cholinephosphotransferase
- CREB:
-
CAMP response elements and protein-CRE binding
- CRY:
-
Cryptochrome
- DAG:
-
Diacylglycerol
- DD:
-
Constant darkness
- DEX:
-
Dexamethasone
- DGL:
-
Diacylglycerol lipase
- DHA:
-
Docosahexaenoic acid (DHA, 22:6n-3)
- DHAP:
-
Dihydroxyacetone phosphate
- EK:
-
Ethanolamine kinase
- EPT:
-
1,2‐Diacylglycerol ethanolaminephosphotransferase
- ER:
-
Endoplasmic reticulum;
- FA:
-
Fatty acid
- G3P:
-
Glycerol-3-phosphate
- GBM:
-
Glioblastoma multiforme
- GCL:
-
Ganglion cell layer
- GPAT:
-
G3P acyltransferase;
- GPL:
-
Glycerophospholipids
- GR:
-
Glucocorticoid receptors
- GRE:
-
Glucocorticoid response elements
- HIF1 α:
-
Hypoxia-inducible factor 1α
- INL:
-
Inner nuclear layer cells
- iPLA2:
-
Calcium-independent phospholipase A2
- L/D:
-
Light dark cycle
- LD:
-
Lipid droplet
- LL:
-
Constant light
- LPA:
-
Lysophosphatidic acid
- LPAAT:
-
Lysophosphatidic acid acyltransferase
- LPCAT:
-
Lysophosphatidylcholine acyltransferase
- LPEAT:
-
Lysophosphatidylethanolamine acyltransferase
- LPIAT:
-
Lysophosphatidylinositol acyltransferase
- LPLAT:
-
Lysophospholipid acyltransferase
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NPAS2:
-
PAS domain-containing protein 2
- NR:
-
Nuclear receptor
- ONL:
-
Outer plexiform layer
- OS:
-
Outer segments
- PA:
-
Phosphatidic acid
- PAP:
-
Phosphatidate phosphohydrolase
- PAP-2:
-
Phosphate phosphatase
- PARP-1:
-
Poly (ADP-ribose) polymerase 1;
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PEMT:
-
Phosphatidylethanolamine methyl transferase
- PER:
-
Period
- PGC-1α:
-
Peroxisome proliferator-activated receptor gamma coactivator1-alpha
- PI:
-
Phosphatidylinositol
- PLA2:
-
Phospholipases A2
- PPAR:
-
Peroxisome proliferator-activated receptor
- PPRE:
-
PPAR regulatory element
- PRX:
-
Peroxiredoxin
- PS:
-
Phosphatidylserine
- ROR:
-
Retinoic acid receptor-related orphan receptor
- RORE:
-
Retinoic acid receptor-related orphan receptor response element
- ROS:
-
Reactive oxygen species
- SCN:
-
Suprachiasmatic nuclei
- SIRT1:
-
NAD+ -dependent deacetylase
- SPM:
-
Sphingomyelin
- SRE:
-
Serum response element
- SREBP:
-
Sterol regulatory element-binding protein
- SRF:
-
Serum response factor
- TAG:
-
Triacylglycerol
- TTFL:
-
Transcriptional–translational feedback loop
References
Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418:935–941. https://doi.org/10.1038/nature00965
Giebultowicz J, Dunlap JC, Loros JJ, Decoursey PJ (2004) No title. Narnia
Dibner C, Schibler U, Albrecht U (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72:517–549. https://doi.org/10.1146/annurev-physiol-021909-135821
Guido ME, Goguen D, De Guido L et al (1999) Circadian and photic regulation of immediate-early gene expression in the hamster suprachiasmatic nucleus. Neuroscience 90:555–571. https://doi.org/10.1016/S0306-4522(98)00467-9
Golombek DA, Rosenstein RE (2010) Physiology of circadian entrainment. Physiol Rev 90:1063–1102. https://doi.org/10.1152/physrev.00009.2009
Moore RY (2013) The suprachiasmatic nucleus and the circadian timing system. In: Progress in molecular biology and translational science. Elsevier B.V., pp 1–28
Takahashi JS, Hong H-K, Ko CH, McDearmon EL (2008) The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9:764–775. https://doi.org/10.1038/nrg2430
Mohawk JA, Green CB, Takahashi JS (2012) Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35:445–462. https://doi.org/10.1146/annurev-neuro-060909-153128
Bass J, Takahashi JS (2010) Circadian integration of metabolism and energetics. Science 330:1349–1354. https://doi.org/10.1126/science.1195027
Guido ME, Garbarino-Pico E, Contin MA et al (2010) Inner retinal circadian clocks and non-visual photoreceptors: novel players in the circadian system. Prog Neurobiol 92:484–504. https://doi.org/10.1016/J.PNEUROBIO.2010.08.005
Buhr ED, Takahashi JS (2013) Molecular components of the mammalian circadian clock. In: Handbook of experimental pharmacology. Howard Hughes Medical Institute, pp 3–27
Bunger MK, Wilsbacher LD, Moran SM et al (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103:1009–1017
Gekakis N, Staknis D, Nguyen HB et al (1998) Role of the CLOCK protein in the mammalian circadian mechanism. Sci 280:1564–1569. https://doi.org/10.1126/science.280.5369.1564
Reick M, Garcia JA, Dudley C, McKnight SL (2001) NPAS2: An analog of clock operative in the mammalian forebrain. Sci 293:506–509. https://doi.org/10.1126/science.1060699
Griffin Jr EA, Staknis D, Weitz CJ et al (1999) Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Sci 286:768–771. https://doi.org/10.1126/science.286.5440.768
Kume K, Zylka MJ, Sriram S et al (1999) mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98:193–205. https://doi.org/10.1016/s0092-8674(00)81014-4
Lee C, Etchegaray JP, Cagampang FR et al (2001) Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107:855–867
Sato TK, Yamada RG, Ukai H et al (2006) Feedback repression is required for mammalian circadian clock function. Nat Genet 38:312–319. https://doi.org/10.1038/ng1745
Bollinger T, Schibler U (2014) Circadian rhythms – from genes to physiology and disease. Swiss Med Wkly 144:w13984. https://doi.org/10.4414/smw.2014.13984
Eide EJ, Woolf MF, Kang H et al (2005) Control of mammalian circadian rhythm by CKI-regulated proteasome-mediated PER2 degradation. Mol Cell Biol 25:2795–2807. https://doi.org/10.1128/MCB.25.7.2795-2807.2005
Godinho SIH, Maywood ES, Shaw L et al (2007) The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Sci 316:897–900. https://doi.org/10.1126/science.1141138
Shirogane T, Jin J, Ang XL, Harper JW (2005) SCFβ-TRCP controls clock-dependent transcription via casein kinase 1-dependent degradation of the mammalian period-1 (Per1) protein. J Biol Chem 280:26863–26872. https://doi.org/10.1074/jbc.M502862200
Siepka SM, Yoo S-H, Park J et al (2007) Circadian mutant overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 129:1011–1023. https://doi.org/10.1016/j.cell.2007.04.030
Yoo S-H, Mohawk JA, Siepka SM et al (2013) Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell 152:1091–1105. https://doi.org/10.1016/j.cell.2013.01.055
Crumbley C, Wang Y, Kojetin DJ, Burris TP (2010) Characterization of the core mammalian clock component, NPAS2, as a REV-ERBα/RORα target gene. J Biol Chem 285:35386–35392. https://doi.org/10.1074/jbc.M110.129288
Preitner N, Damiola F, Lopez-Molina L et al (2002) The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110:251–260
Adamovich Y, Aviram R, Asher G (2015) The emerging roles of lipids in circadian control. Biochim. Biophys Acta - Mol Cell Biol Lipids 1851:1017–1025
Yang X, Downes M, Yu RT et al (2006) Nuclear receptor expression links the circadian clock to metabolism. Cell 126:801–810. https://doi.org/10.1016/j.cell.2006.06.050
Sato TK, Panda S, Miraglia LJ et al (2004) A functional genomics strategy reveals rora as a component of the mammalian circadian clock. Neuron 43:527–537. https://doi.org/10.1016/j.neuron.2004.07.018
Akashi M, Takumi T (2005) The orphan nuclear receptor RORα regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol 12:441–448. https://doi.org/10.1038/nsmb925
Takeda Y, Kang HS, Angers M, Jetten AM (2011) Retinoic acid-related orphan receptor γ directly regulates neuronal PAS domain protein 2 transcription in vivo. Nucleic Acids Res 39:4769–4782. https://doi.org/10.1093/nar/gkq1335
Crumbley C, Burris TP (2011) Direct regulation of CLOCK expression by REV-ERB. PLoS ONE 6:e17290. https://doi.org/10.1371/journal.pone.0017290
Panda S, Antoch MP, Miller BH et al (2002) Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109:307–320
Hatanaka F, Matsubara C, Myung J et al (2010) Genome-wide profiling of the core clock protein BMAL1 targets reveals a strict relationship with metabolism. Mol Cell Biol 30:5636–5648. https://doi.org/10.1128/MCB.00781-10
Rey G, Cesbron F, Rougemont J et al (2011) Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol 9:e1000595. https://doi.org/10.1371/journal.pbio.1000595
Sulli G, Lam MTY, Panda S (2019) Interplay between circadian clock and cancer: new frontiers for cancer treatment. Trends in Cancer 5:475–494
Green CB, Takahashi JS, Bass J (2008) The meter of metabolism. Cell 134:728–742. https://doi.org/10.1016/j.cell.2008.08.022
Huang X-L, Fu C-J, Bu R-F (2011) Role of circadian clocks in the development and therapeutics of cancer. J Int Med Res 39:2061–2066. https://doi.org/10.1177/147323001103900601
Huang W, Ramsey KM, Marcheva B, Bass J (2011) Circadian rhythms, sleep, and metabolism. J Clin Invest 121:2133–2141. https://doi.org/10.1172/JCI46043
Laposky AD, Bass J, Kohsaka A, Turek FW (2008) Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett 582:142–151. https://doi.org/10.1016/j.febslet.2007.06.079
Kubo T, Ozasa K, Mikami K et al (2006) Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol 164:549–555. https://doi.org/10.1093/aje/kwj232
Masri S, Sassone-Corsi P (2018) The emerging link between cancer, metabolism, and circadian rhythms. Nat Med 24:1795–1803
Edgar RS, Green E10.1038/nature11088https://www.nature.com/articles/nature11088#supplementary-informationW, Zhao Y, et al (2012) Peroxiredoxins are conserved markers of circadian rhythms. Nature 485:459.
O’Neill JS, van Ooijen G, Dixon LE, et al (2011) Circadian rhythms persist without transcription in a eukaryote. Nature 469:554. 10.1038/nature09654https://www.nature.com/articles/nature09654#supplementary-informationf
Olmedo M, O’Neill JS, Edgar RS et al (2012) Circadian regulation of olfaction and an evolutionarily conserved, nontranscriptional marker in Caenorhabditis elegans. Proc Natl Acad Sci U S A 109:20479–20484. https://doi.org/10.1073/pnas.1211705109
O’Neill JS, Reddy AB (2011) Circadian clocks in human red blood cells. Nature 469:498–503. https://doi.org/10.1038/nature09702
Wagner PM, Sosa Alderete LG, Gorné LD et al (2018) Proliferative glioblastoma cancer cells exhibit persisting temporal control of metabolism and display differential temporal drug susceptibility in chemotherapy. Mol Neurobiol. https://doi.org/10.1007/s12035-018-1152-3
Wang M, Jiang Y-Y, Kim KM et al (2011) A universal molecular clock of protein folds and its power in tracing the early history of aerobic metabolism and planet oxygenation. Mol Biol Evol 28:567–582. https://doi.org/10.1093/molbev/msq232
Crowe SA, Døssing LN, Beukes NJ, et al (2013) Atmospheric oxygenation three billion years ago. Nature 501:535. 10.1038/nature12426https://www.nature.com/articles/nature12426#supplementary-information
Causton HC, Feeney KA, Ziegler CA, O’Neill JS (2015) Metabolic cycles in yeast share features conserved among circadian rhythms. Curr Biol 25:1056–1062. https://doi.org/10.1016/j.cub.2015.02.035
Rey G, Reddy AB (2013) Connecting cellular metabolism to circadian clocks. Trends Cell Biol 23:234–241. https://doi.org/10.1016/j.tcb.2013.01.003
Ramsey KM, Yoshino J, Brace CS et al (2009) Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324:651–654. https://doi.org/10.1126/science.1171641
Nakahata Y, Sahar S, Astarita G et al (2009) Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324:654–657. https://doi.org/10.1126/science.1170803
Rutter J, Reick M, Wu LC, McKnight SL (2001) Regulation of clock and NPAS2 DNA binding by the Redox state of NAD cofactors. Sci 293:510. https://doi.org/10.1126/science.1060698
Gupta N, Ragsdale SW (2011) Thiol-disulfide redox dependence of heme binding and heme ligand switching in nuclear hormone receptor rev-erbβ. J Biol Chem 286:4392–4403. https://doi.org/10.1074/jbc.M110.193466
Asher G, Gatfield D, Stratmann M et al (2008) SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134:317–328. https://doi.org/10.1016/j.cell.2008.06.050
Nakahata Y, Kaluzova M, Grimaldi B et al (2008) The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134:329–340. https://doi.org/10.1016/j.cell.2008.07.002
Aguilar-Arnal L, Ranjit S, Stringari C et al (2016) Spatial dynamics of SIRT1 and the subnuclear distribution of NADH species. Proc Natl Acad Sci U S A 113:12715–121720. https://doi.org/10.1073/pnas.1609227113
Ramadori G, Fujikawa T, Fukuda M et al (2010) SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab 12:78–87. https://doi.org/10.1016/j.cmet.2010.05.010
Asher G, Reinke H, Altmeyer M et al (2010) Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell 142:943–953. https://doi.org/10.1016/j.cell.2010.08.016
Um JH, Pendergast JS, Springer DA, et al (2011) AMPK regulates circadian rhythms in a tissue- and isoform-specific manner. PLoS One 6:. https://doi.org/10.1371/journal.pone.0018450
Jordan SD, Lamia KA (2013) AMPK at the crossroads of circadian clocks and metabolism. Mol Cell Endocrinol 366:163–169
Lamia KA, Sachdeva UM, DiTacchio L et al (2009) AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Sci 326:437–440. https://doi.org/10.1126/science.1172156
Jee HU, Yang S, Yamazaki S et al (2007) Activation of 5′-AMP-activated kinase with diabetes drug metformin induces casein kinase Iε (CKIε)-dependent degradation of clock protein mPer2. J Biol Chem 282:20794–20798. https://doi.org/10.1074/jbc.C700070200
Xue B, Kahn BB (2006) AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol 574:73–83
Polekhina G, Gupta A, Michell BJ et al (2003) AMPK β subunit targets metabolic stress sensing to glycogen. Curr Biol 13:867–871. https://doi.org/10.1016/S0960-9822(03)00292-6
Kohsaka A, Laposky AD, Ramsey KM et al (2007) High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6:414–421. https://doi.org/10.1016/j.cmet.2007.09.006
Lee Y, Kim EK (2013) AMP-activated protein kinase as a key molecular link between metabolism and clockwork. Exp Mol Med 45:33
Puig LS, Valera-Alberni M, Cantó C, Pillon NJ (2018) Circadian rhythms and mitochondria: connecting the dots. Front. Genet. 9
Vaughan M, Jordan SD, Duglan D et al (2019) Phosphorylation of CRY1 serine 71 alters voluntary activity but not circadian rhythms in vivo. J Biol Rhythms 34:401–409. https://doi.org/10.1177/0748730419858525
Masri S, Rigor P, Cervantes M et al (2014) Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 158:659–672. https://doi.org/10.1016/j.cell.2014.06.050
O’Neill JS, Maywood ES, Chesham JE et al (2008) cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science 320:949–953. https://doi.org/10.1126/science.1152506
Hirota T, Kon N, Itagaki T et al (2010) Transcriptional repressor TIEG1 regulates Bmal1 gene through GC box and controls circadian clockwork. Genes Cells 15:111–121. https://doi.org/10.1111/j.1365-2443.2009.01371.x
Hoyle NP, O’Neill JS (2015) Oxidation-reduction cycles of peroxiredoxin proteins and nontranscriptional aspects of timekeeping. Biochemistry 54:184–193. https://doi.org/10.1021/bi5008386
Reddy AB, Rey G (2014) Metabolic and nontranscriptional circadian clocks: eukaryotes. Annu Rev Biochem 83:165–189. https://doi.org/10.1146/annurev-biochem-060713-035623
Peek CB, Levine DC, Cedernaes J et al (2017) Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab 25:86–92. https://doi.org/10.1016/j.cmet.2016.09.010
Adamovich Y, Ladeuix B, Golik M et al (2017) Rhythmic oxygen levels reset circadian clocks through HIF1α. Cell Metab 25:93–101. https://doi.org/10.1016/j.cmet.2016.09.014
Adamovich Y, Ladeuix B, Sobel J et al (2019) Oxygen and carbon dioxide rhythms are circadian clock controlled and differentially directed by behavioral signals. Cell Metab 29:1092-1103.e3. https://doi.org/10.1016/j.cmet.2019.01.007
Wu Y, Tang D, Liu N et al (2017) Reciprocal regulation between the circadian clock and hypoxia signaling at the genome level in mammals. Cell Metab 25:73–85. https://doi.org/10.1016/j.cmet.2016.09.009
Storch K-F, Lipan O, Leykin I et al (2002) Extensive and divergent circadian gene expression in liver and heart. Nature 417:78–83. https://doi.org/10.1038/nature744
Hughes ME, DiTacchio L, Hayes KR et al (2009) Harmonics of circadian gene transcription in mammals. PLoS Genet 5:e1000442. https://doi.org/10.1371/journal.pgen.1000442
Ueda HR, Chen W, Adachi A et al (2002) A transcription factor response element for gene expression during circadian night. Nature 418:534–539. https://doi.org/10.1038/nature00906
Keller M, Mazuch J, Abraham U et al (2009) A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A 106:21407–21412. https://doi.org/10.1073/pnas.0906361106
Vollmers C, Gill S, DiTacchio L et al (2009) Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A 106:21453–21458. https://doi.org/10.1073/pnas.0909591106
Hoogerwerf WA, Sinha M, Conesa A et al (2008) Transcriptional profiling of mRNA expression in the mouse distal colon. Gastroenterology 135:2019–2029. https://doi.org/10.1053/j.gastro.2008.08.048
Zhang R, Lahens NF, Ballance HI et al (2014) A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci U S A 111:16219–16224. https://doi.org/10.1073/pnas.1408886111
Cagampang FRA, Sheward WJ, Harmar AJ et al (1998) Circadian changes in the expression of vasoactive intestinal peptide 2 receptor mRNA in the rat suprachiasmatic nuclei. Mol Brain Res 54:108–112. https://doi.org/10.1016/S0169-328X(97)00327-6
Chen YG, Mantalaris A, Bourne P et al (2000) Expression of mPer1 and mPer2, two mammalian clock genes, in murine bone marrow. Biochem Biophys Res Commun 276:724–728. https://doi.org/10.1006/bbrc.2000.3536
Yamamoto T, Nakahata Y, Soma H, et al (2004) Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol 5:. https://doi.org/10.1186/1471-2199-5-18
Liu S, Cai Y, Sothern RB et al (2007) Chronobiological analysis of circadian patterns in transcription of seven key clock genes in six peripheral tissues in mice. Chronobiol Int 24:793–820. https://doi.org/10.1080/07420520701672556
Zhu B, Zhang Q, Pan Y et al (2017) A cell-autonomous mammalian 12 hr clock coordinates metabolic and stress rhythms. Cell Metab 25:1305-1319.e9. https://doi.org/10.1016/J.CMET.2017.05.004
Ayala DE, Hermida RC, Garciat L et al (1990) Multiple component analysis of plasma growth hormone in children with standard and short stature. Chronobiol Int 7:217–220. https://doi.org/10.3109/07420529009056977
Bjerner B, Holm A, Swesson A (1955) Diurnal variation in mental performance; a study of three-shift workers. Br J Ind Med 12:103–110. https://doi.org/10.1136/oem.12.2.103
Broughton R, Mullington J (1992) Circasemidian sleep propensity and the phase-amplitude maintenance model of human sleep/wake regulation. J Sleep Res 1:93–98. https://doi.org/10.1111/j.1365-2869.1992.tb00017.x
Lamia KA, Papp SJ, Yu RT et al (2011) Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480:552–556. https://doi.org/10.1038/nature10700
Schmutz I, Ripperger JA, Baeriswyl-Aebischer S, Albrecht U (2010) The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev 24:345–357. https://doi.org/10.1101/gad.564110
Burris TP (2008) Nuclear hormone receptors for heme: REV-ERBα and REV-ERBβ are ligand-regulated components of the mammalian clock. Mol Endocrinol 22:1509–1520. https://doi.org/10.1210/me.2007-0519
Zvonic S, Ptitsyn AA, Conrad SA et al (2006) Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55:962–970. https://doi.org/10.2337/diabetes.55.04.06.db05-0873
Torra IP, Tsibulsky V, Delaunay F et al (2000) Circadian and glucocorticoid regulation of Rev-erbα expression in liver. Endocrinol 141:3799–3806. https://doi.org/10.1210/endo.141.10.7708
Ramakrishnan SN, Muscat GEO (2006) The orphan Rev-Erb nuclear receptors: a link between metabolism, circadian rhythm and inflammation? Nucl Recept Signal 4:nrs.04009. https://doi.org/10.1621/nrs.04009
Raghuram S, Stayrook KR, Huang P et al (2007) Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat Struct Mol Biol 14:1207–1213. https://doi.org/10.1038/nsmb1344
Raspé E, Duez H, Mansén A et al (2002) Identification of Rev-erbα as a physiological repressor of apoC-III gene transcription. J Lipid Res 43:2172–2179. https://doi.org/10.1194/jlr.M200386-JLR200
Raspé E, Duez H, Gervois P et al (2001) Transcriptional regulation of apolipoprotein C-III gene expression by the orphan nuclear receptor RORα. J Biol Chem 276:2865–2871. https://doi.org/10.1074/jbc.M004982200
Vu-Dac N, Chopin-Delannoy S, Gervois P et al (1998) The nuclear receptors peroxisome proliferator-activated receptor α and rev-erbα mediate the species-specific regulation of apolipoprotein A-I expression by fibrates. J Biol Chem 273:25713–25720. https://doi.org/10.1074/jbc.273.40.25713
Ramakrishnan SN, Lau P, Burke LJ, Muscat GEO (2005) Rev-erbβ regulates the expression of genes involved in lipid absorption in skeletal muscle cells: Evidence for cross-talk between orphan nuclear receptors and myokines. J Biol Chem 280:8651–8659. https://doi.org/10.1074/jbc.M413949200
Yin L, Wu N, Curtin JC et al (2007) Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Sci 318:1786–1789. https://doi.org/10.1126/science.1150179
Delezie J, Dumont S, Dardente H et al (2012) The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J 26:3321–3335. https://doi.org/10.1096/fj.12-208751
Kang HS, Angers M, Beak JY et al (2007) Gene expression profiling reveals a regulatory role for RORα and RORγ in phase I and phase II metabolism. Physiol Genomics 31:281–294. https://doi.org/10.1152/physiolgenomics.00098.2007
Fontaine C, Dubois G, Duguay Y et al (2003) The orphan nuclear receptor Rev-Erbα is a peroxisome proliferator-activated receptor (PPAR) γ target gene and promotes PPARγ-induced adipocyte differentiation. J Biol Chem 278:37672–37680. https://doi.org/10.1074/jbc.M304664200
Wang J, Lazar MA (2008) Bifunctional role of Rev-erbα in adipocyte differentiation. Mol Cell Biol 28:2213–2220. https://doi.org/10.1128/mcb.01608-07
Chawla A, Lazar MA (1993) Induction of Rev-ErbAα, an orphan receptor encoded on the opposite strand of the α-thyroid hormone receptor gene, during adipocyte differentiation. J Biol Chem 268:16265–16269
Woldt E, Sebti Y, Solt LA et al (2013) Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med 19:1039–1046. https://doi.org/10.1038/nm.3213
Duez H, Staels B (2009) Rev-erb-alpha: an integrator of circadian rhythms and metabolism. J Appl Physiol 107:1972–1980. https://doi.org/10.1152/japplphysiol.00570.2009
Le Martelot G, Claudel T, Gatfield D et al (2009) REV-ERBα participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol 7:e1000181. https://doi.org/10.1371/journal.pbio.1000181
Feng D, Liu T, Sun Z et al (2011) A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Sci 331:1315–1319. https://doi.org/10.1126/science.1198125
S Sen, S Dumont, D Sage-Ciocca, et al (2018) Expression of the clock gene Rev-erbα in the brain controls the circadian organisation of food intake and locomotor activity, but not daily variations of energy metabolism. J Neuroendocrinol 30:. https://doi.org/10.1111/JNE.12557
Cho H, Zhao X, Hatori M et al (2012) Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485:123–127. https://doi.org/10.1038/nature11048
Solt LA, Wang Y, Banerjee S et al (2012) Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485:62–68. https://doi.org/10.1038/nature11030
Scheiermann C, Kunisaki Y, Frenette PS (2013) Circadian control of the immune system. Nat Rev Immunol 13:190–198. https://doi.org/10.1038/nri3386
Vieira E, Marroquí L, Batista TM et al (2012) The clock gene Rev-erbα regulates pancreatic β-cell function: modulation by leptin and high-fat diet. Endocrinology 153:592–601. https://doi.org/10.1210/en.2011-1595
Sulli G, Rommel A, Wang X et al (2018) Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553:351–355. https://doi.org/10.1038/nature25170
Wagner PM, Monjes NM, Guido ME (2019) Chemotherapeutic Effect of SR9009, a REV-ERB Agonist, on the Human Glioblastoma T98G Cells. ASN Neuro 11:175909141989271. https://doi.org/10.1177/1759091419892713
Welte MA, Gould AP (2017) Lipid droplet functions beyond energy storage. Biochim Biophys Acta - Mol Cell Biol Lipids 1862:1260–1272. https://doi.org/10.1016/J.BBALIP.2017.07.006
Bensaad K, Favaro E, Lewis CA et al (2014) Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep 9:349–365. https://doi.org/10.1016/j.celrep.2014.08.056
Gooley JJ (2016) Circadian regulation of lipid metabolism. In: Proceedings of the Nutrition Society. Cambridge University Press, pp 440–450
Adamovich Y, Rousso-Noori L, Zwighaft Z et al (2014) Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab 19:319–330. https://doi.org/10.1016/j.cmet.2013.12.016
Chua ECP, Shui G, Lee ITG et al (2013) Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc Natl Acad Sci U S A 110:14468–14473. https://doi.org/10.1073/pnas.1222647110
Guo Y, Walther TC, Rao M et al (2008) Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453:657–661. https://doi.org/10.1038/nature06928
Fei W, Shui G, Zhang Y et al (2011) A role for phosphatidic acid in the formation of "supersized" lipid droplets. PLoS Genet 7:e1002201. https://doi.org/10.1371/journal.pgen.1002201
Gorné LD, Acosta-Rodríguez VA, Pasquaré SJ et al (2015) The mouse liver displays daily rhythms in the metabolism of phospholipids and in the activity of lipid synthesizing enzymes. Chronobiol Int 32:11–26. https://doi.org/10.3109/07420528.2014.949734
Gréchez-Cassiau A, Feillet C, Guérin S, Delaunay F (2015) The hepatic circadian clock regulates the choline kinase α gene through the BMAL1-REV-ERBα axis. Chronobiol Int 32:774–784. https://doi.org/10.3109/07420528.2015.1046601
Jetten AM (2009) Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 7
Mamontova A, Séguret-Macé S, Esposito B et al (1998) Severe atherosclerosis and hypoalphalipoproteinemia in the staggerer mouse, a mutant of the nuclear receptor RORα. Circulation 98:2738–2743. https://doi.org/10.1161/01.CIR.98.24.2738
Lau P, Fitzsimmons RL, Raichur S et al (2008) The orphan nuclear receptor, RORα, regulates gene expression that controls lipid metabolism: staggerer (sg/sg) mice are resistant to diet-induced obesity. J Biol Chem 283:18411–18421. https://doi.org/10.1074/jbc.M710526200
Takeda Y, Kang HS, Lih FB et al (2014) Retinoid acid-related orphan receptor γ, RORγ, participates in diurnal transcriptional regulation of lipid metabolic genes. Nucleic Acids Res 42:10448–10459. https://doi.org/10.1093/nar/gku766
Takeda Y, Jothi R, Birault V, Jetten AM (2012) RORγ directly regulates the circadian expression of clock genes and downstream targets in vivo. Nucleic Acids Res 40:8519–8535. https://doi.org/10.1093/nar/gks630
Grygiel-Górniak B (2014) Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications - a review. Nutr J 13:. https://doi.org/10.1186/1475-2891-13-17
Oishi K, Shirai H, Ishida N (2005) CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor α (PPARα) in mice. Biochem J 386:575–581. https://doi.org/10.1042/BJ20041150
Grimaldi B, Bellet MM, Katada S et al (2010) PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab 12:509–520. https://doi.org/10.1016/j.cmet.2010.10.005
Wang N, Yang G, Jia Z et al (2008) Vascular PPARγ controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab 8:482–491. https://doi.org/10.1016/j.cmet.2008.10.009
Yang G, Jia Z, Aoyagi T et al (2012) Systemic PPARγ deletion impairs circadian rhythms of behavior and metabolism. PLoS ONE 7:e38117. https://doi.org/10.1371/journal.pone.0038117
Gervois P, Chopin-Delannoy S, Fadel A et al (1999) Fibrates increase human REV-ERBα expression in liver via a novel peroxisome proliferator-activated receptor response element. Mol Endocrinol 13:400–409. https://doi.org/10.1210/mend.13.3.0248
Fontaine C, Rigamonti E, Pourcet B et al (2008) The nuclear receptor Rev-erbα is a Liver X Receptor (LXR) target gene driving a negative feedback loop on select LXR-induced pathways in human macrophages. Mol Endocrinol 22:1797–1811. https://doi.org/10.1210/me.2007-0439
Alex S, Lange K, Amolo T et al (2013) Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor. Mol Cell Biol 33:1303–1316. https://doi.org/10.1128/mcb.00858-12
Krey G, Braissant O, L’Horset F et al (1997) Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol 11:779–791. https://doi.org/10.1210/mend.11.6.0007
Liberato MV, Nascimento AS, Ayers SD et al (2012) Medium chain fatty acids are selective peroxisome proliferator activated receptor (PPAR) γ activators and Pan-PPAR partial agonists. PLoS ONE 7:36297. https://doi.org/10.1371/journal.pone.0036297
McIntyre TM, Pontsler AV, Silva AR et al (2003) Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARγ agonist. Proc Natl Acad Sci U S A 100:131–136. https://doi.org/10.1073/pnas.0135855100
Eckel-Mahan KL, Patel VR, De Mateo S et al (2013) Reprogramming of the circadian clock by nutritional challenge. Cell 155:1464–1478. https://doi.org/10.1016/j.cell.2013.11.034
Foster DW (2012) Malonyl-CoA: The regulator of fatty acid synthesis and oxidation. J Clin Invest 122:1958–1959
Rivera-Zavala JB, Molina-Aguilar C, Pérez-Mendoza M et al (2017) Daytime restricted feeding modifies the daily regulation of fatty acid β-oxidation and the lipoprotein profile in rats. Br J Nutr 117:930–941. https://doi.org/10.1017/S0007114517000800
Li MD, Li CM, Wang Z (2012) The role of circadian clocks in metabolic disease. Yale J Biol Med 85:387–401
Finck BN, Kelly DP (2006) PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J Clin Invest 116:615–622
Estall JL, Kahn M, Cooper MP et al (2009) Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-γ coactivator-1α expression. Diabetes 58:1499–1508. https://doi.org/10.2337/db08-1571
Gooley JJ, Chua EC-P (2014) Diurnal regulation of lipid metabolism and applications of circadian lipidomics. J Genet Genomics 41:231–250. https://doi.org/10.1016/j.jgg.2014.04.001
Takeuchi K, Reue K (2009) Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab
Hishikawa D, Hashidate T, Shimizu T, Shindou H (2014) Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J Lipid Res 55:799–807
Kennedy EP, Weiss SB (1956) The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem 222:193–214
McMaster CR (2018) From yeast to humans – roles of the Kennedy pathway for phosphatidylcholine synthesis. FEBS Lett 592:1256–1272
Gibellini F, Smith TK (2010) The Kennedy pathway-de novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 62:414–428
Reue K, Wang H (2019) Mammalian lipin phosphatidic acid phosphatases in lipid synthesis and beyond: metabolic and inflammatory disorders. J Lipid Res 60:728–733
Lee J, Ridgway ND (2020) Substrate channeling in the glycerol-3-phosphate pathway regulates the synthesis, storage and secretion of glycerolipids. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1865
Li Z, Vance DE (2008) Phosphatidylcholine and choline homeostasis. J Lipid Res 49:1187–1194
Kol M, Panatala R, Nordmann M et al (2016) Switching head group selectivity in mammalian sphingolipid biosynthesis by active-site engineering of sphingomyelin synthases. J Lipid Res 57:1273–1285. https://doi.org/10.1194/jlr.M068692
Dowhan W, Bogdanov M (2009) Lipid-dependent membrane protein topogenesis. Annu Rev Biochem 78:515–540
Van Meer G, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9:112–124
Niebergall LJ, Vance DE (2012) The ratio of phosphatidylcholine to phosphatidylethanolamine does not predict integrity of growing MT58 Chinese hamster ovary cells. Biochim Biophys Acta - Mol Cell Biol Lipids 1821:324–334. https://doi.org/10.1016/j.bbalip.2011.10.018
van der Veen JN, Kennelly JP, Wan S et al (2017) The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta - Biomembr 1859:1558–1572
Kim S-J, Tang T, Abbott M et al (2016) AMPK phosphorylates desnutrin/ATGL and hormone-sensitive lipase to regulate lipolysis and fatty acid oxidation within adipose tissue. Mol Cell Biol 36:1961–1976. https://doi.org/10.1128/mcb.00244-16
Fiume R, Faenza I, Sheth B, et al (2019) Nuclear phosphoinositides: their regulation and roles in nuclear functions. Int J Mol Sci 20:. https://doi.org/10.3390/ijms20122991
Harayama T, Riezman H (2018) Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Biol 19:281–296
Acosta-Rodríguez VA, Márquez S, Salvador GA et al (2013) Daily rhythms of glycerophospholipid synthesis in fibroblast cultures involve differential enzyme contributions. J Lipid Res 54:1798–1811. https://doi.org/10.1194/jlr.M034264
Balsalobre A, Damiola F, Schibler U (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93:929–937
Marquez S, Crespo P, Carlini V et al (2004) The metabolism of phospholipids oscillates rhythmically in cultures of fibroblasts and is regulated by the clock protein PERIOD 1. FASEB J 18:519–521. https://doi.org/10.1096/fj.03-0417fje
Nagoshi E, Brown SA, Dibner C et al (2005) Circadian gene expression in cultured cells. Methods Enzymol 393:543–557. https://doi.org/10.1016/S0076-6879(05)93028-0
Guido ME, Marchese NA, Rios MN et al (2020) Non-visual opsins and novel photo-detectors in the vertebrate inner retina mediate light responses within the blue spectrum region. Cell Mol Neurobiol. https://doi.org/10.1007/S10571-020-00997-X
DM Verra, BS Sajdak, DK Merriman, D Hicks (2020) Diurnal rodents as pertinent animal models of human retinal physiology and pathology. Prog Retin Eye Res 74:. https://doi.org/10.1016/J.PRETEYERES.2019.100776
Wylie DR, Gutiérrez-Ibáñez C, Gaede AH et al (2018) Visual-cerebellar pathways and their roles in the control of avian flight. Front Neurosci 0:223. https://doi.org/10.3389/FNINS.2018.00223
Guido ME, Pico EG, Caputto BL (2001) Circadian regulation of phospholipid metabolism in retinal photoreceptors and ganglion cells. J Neurochem 76:835–845. https://doi.org/10.1046/j.1471-4159.2001.00081.x
Garbarino-Pico E, Carpentieri AR, Contin MA, et al (2004) Retinal ganglion cells are autonomous circadian oscillators synthesizing N-acetylserotonin during the day*. https://doi.org/10.1074/jbc.M309248200
Garbarino-Pico E, Valdez DJ, Contín MA et al (2005) Rhythms of glycerophospholipid synthesis in retinal inner nuclear layer cells. Neurochem Int 47:260–270. https://doi.org/10.1016/J.NEUINT.2005.04.024
Guido ME, Caputto BL (1990) Labeling of retina and optic tectum phospholipids in chickens exposed to light or dark. J Neurochem 55:1855–1860. https://doi.org/10.1111/j.1471-4159.1990.tb05768.x
Felder-SchmittbuhlBuhrDkhissi-Benyahya MPEDO et al (2018) Ocular clocks: adapting mechanisms for eye functions and health. Invest Ophthalmol Vis Sci 59:4856–4870. https://doi.org/10.1167/IOVS.18-24957
McMahon DG, Iuvone PM, Tosini G (2014) Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res 39:58–76. https://doi.org/10.1016/J.PRETEYERES.2013.12.001
Besharse JC, McMahon DG (2016) The retina and other light sensitive ocular clocks. J Biol Rhythms 31:223. https://doi.org/10.1177/0748730416642657
Garbarino-Pico E, Carpentieri AR, Castagnet PI et al (2004) Synthesis of retinal ganglion cell phospholipids is under control of an endogenious circadian clock: daily variations in phospholipid-synthesizing enzyme activities. J Neurosci Res 76:642–652. https://doi.org/10.1002/jnr.20126
Valdez DJ, Garbarino-Pico E, Diaz NM et al (2012) Differential regulation of arylalkylamine N-acetyltransferase activity in chicken retinal ganglion cells by light and circadian clock. Chronobiol Int 29:1011–1020. https://doi.org/10.3109/07420528.2012.707160
Haque R, Chong NW, Ali F et al (2011) Melatonin synthesis in retina: cAMP-dependent transcriptional regulation of chicken arylalkylamine N-acetyltransferase by a CRE-like sequence and a TTATT repeat motif in the proximal promoter. J Neurochem 119:6–17. https://doi.org/10.1111/J.1471-4159.2011.07397.X
Caputto BL, Guido ME (2000) Immediate early gene expression within the visual system: Light and circadian regulation in the retina and the suprachiasmatic nucleus. Neurochem Res 25:153–162. https://doi.org/10.1023/A:1007508020173
Bazan NG (2018) Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol Aspects Med 64:18–33
Bussolino DF, De Arriba Zerpa GA, Grabois VR et al (1998) Light affects c-fos expression and phospholipid synthesis in both retinal ganglion cells and photoreceptor cells in an opposite way for each cell type. Mol Brain Res 58:10–15. https://doi.org/10.1016/S0169-328X(98)00065-5
Guido ME, de Arriba Zerpa GA, Bussolino DF, Caputto BL (1996) Immediate early gene c-fos regulates the synthesis of phospholipids but not of gangliosides. J Neurosci Res 43:93–98. https://doi.org/10.1002/jnr.490430112
Giusto NM, Pasquaré SJ, Salvador GA, De Boschero MGI (2010) Lipid second messengers and related enzymes in vertebrate rod outer segments. J Lipid Res 51:685–700
Bussolino DF, Guido ME, Gil GA et al (2001) c-Fos associates with the endoplasmic reticulum and activates phospholipid metabolism. FASEB J 15:556–558. https://doi.org/10.1096/fj.00-0446fje
Giusto NM, Pasquaré SJ, Salvador GA et al (2000) Lipid metabolism in vertebrate retinal rod outer segments. Prog Lipid Res 39:315–391
De Arriba Zerpa GA, Guido ME, Bussolino DF et al (1999) Light exposure activates retina ganglion cell lysophosphatidic acid acyl transferase and phosphatidic acid phosphatase by a c-fos-dependent mechanism. J Neurochem 73:1228–1235. https://doi.org/10.1046/j.1471-4159.1999.0731228.x
Asher G, Schibler U (2011) Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab 13:125–137
Eckel-Mahan K, Sassone-Corsi P (2013) Metabolism and the circadian clock converge. Physiol Rev 93:107–135. https://doi.org/10.1152/physrev.00016.2012
Nagoshi E, Saini C, Bauer C et al (2004) Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 119:693–705. https://doi.org/10.1016/j.cell.2004.11.015
Turek FW, Joshu C, Kohsaka A et al (2005) Obesity and metabolic syndrome in circadian clock mutant mice. Sci 308:1043–1045. https://doi.org/10.1126/science.1108750
Bray MS, Young ME (2011) Regulation of fatty acid metabolism by cell autonomous circadian clocks: Time to fatten up on information? J Biol Chem 286:11883–11889
Shindou H, Hishikawa D, Harayama T et al (2013) Generation of membrane diversity by lysophospholipid acyltransferases. J Biochem 154:21–28
Mouchlis VD, Dennis EA (2016) Membrane and inhibitor interactions of intracellular phospholipases A2. Adv Biol Regul 61:17–24
Fisher AB (2018) The phospholipase A2 activity of peroxiredoxin 6. J Lipid Res 59:1132–1147
Kawana H, Kano K, Shindou H et al (2019) An accurate and versatile method for determining the acyl group-introducing position of lysophospholipid acyltransferases. Biochim Biophys Acta - Mol Cell Biol Lipids 1864:1053–1060. https://doi.org/10.1016/j.bbalip.2019.02.008
Wang B, Tontonoz P (2019) Phospholipid remodeling in physiology and disease. Annu Rev Physiol 81:165–188
Gaglio D, Metallo CM, Gameiro PA et al (2014) Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol 7:523–523. https://doi.org/10.1038/msb.2011.56
Beloribi-Djefaflia S, Vasseur S, Guillaumond F (2016) Lipid metabolic reprogramming in cancer cells. Oncogenesis 5:e189–e189. https://doi.org/10.1038/oncsis.2015.49
Lacal JC, Campos JM (2015) Preclinical characterization of RSM-932A, a novel anticancer drug targeting the human choline kinase alpha, an enzyme involved in increased lipid metabolism of cancer cells. Mol Cancer Ther 14:31–39. https://doi.org/10.1158/1535-7163.MCT-14-0531
Nomura DK, Long JZ, Niessen S et al (2010) Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 140:49–61. https://doi.org/10.1016/j.cell.2009.11.027
Dyar KA, Lutter D, Artati A et al (2018) Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell 174:1571-1585.e11. https://doi.org/10.1016/j.cell.2018.08.042
Gallego-Ortega D, Gómez del Pulgar T, Valdés-Mora F et al (2011) Involvement of human choline kinase alpha and beta in carcinogenesis: a different role in lipid metabolism and biological functions. Adv Enzyme Regul 51:183–194
Wagner PM, Prucca CG, Velazquez FN et al (2021) Temporal regulation of tumor growth in nocturnal mammals: In vivo studies and chemotherapeutical potential. FASEB J 35:e21231. https://doi.org/10.1096/fj.202001753R
Kent C (2005) Regulatory enzymes of phosphatidylcholine biosynthesis: a personal perspective. Biochim. Biophys Acta - Mol Cell Biol Lipids 1733:53–66
Zhanfeng N, Yanhui L, Zhou F, et al (2015) Circadian genes Per1 and Per2 increase radiosensitivity of glioma in vivo. Oncotarget 6:9951–8. https://doi.org/10.18632/oncotarget.3179
Devaux PF (1991) Static and dynamic lipid asymmetry in cell membranes. Biochemistry 30:1163–1173. https://doi.org/10.1021/bi00219a001
Listenberger LL, Han X, Lewis SE et al (2003) Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A 100:3077–3082. https://doi.org/10.1073/pnas.0630588100
Pol A, Gross SP, Parton RG (2014) Biogenesis of the multifunctional lipid droplet: Lipids, proteins, and sites. J Cell Biol 204:635–646. https://doi.org/10.1083/jcb.201311051
Hafez IM, Cullis PR (2001) Roles of lipid polymorphism in intracellular delivery. Adv Drug Deliv Rev 47:139–148. https://doi.org/10.1016/S0169-409X(01)00103-X
Ren J, Pulakat L, Whaley-Connell A, Sowers JR (2010) Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med 88:993–1001
Supale S, Li N, Brun T, Maechler P (2012) Mitochondrial dysfunction in pancreatic β cells. Trends Endocrinol Metab 23:477–487
Johri A, Beal MF (2012) Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther 342:619–630
Baysal BE, Ferrell RE, Willett-Brozick JE et al (2000) Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Sci 287:848–851. https://doi.org/10.1126/science.287.5454.848
Campos SS, Diez GR, Oresti GM, Salvador GA (2015) Dopaminergic neurons respond to iron-induced oxidative stress by modulating lipid acylation and deacylation cycles. PLoS One 10:. https://doi.org/10.1371/journal.pone.0130726
Rodríguez Diez G, Sánchez Campos S, Giusto NM, Salvador GA (2013) Specific roles for Group V secretory PLA2 in retinal iron-induced oxidative stress. Implications for age-related macular degeneration. Exp Eye Res 113:172–181. https://doi.org/10.1016/j.exer.2013.05.019
Fisher AB (2017) Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch Biochem Biophys 617:68–83
Putker M, Crosby P, Feeney KA et al (2018) Mammalian circadian period, but not phase and amplitude, is robust against redox and metabolic perturbations. Antioxidants Redox Signal 28:507–520. https://doi.org/10.1089/ars.2016.6911
Zhang Y, Fang B, Emmett MJ et al (2015) Gene regulation. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science 348:1488–1492. https://doi.org/10.1126/science.aab3021
Dibner C, Schibler U (2015) Circadian timing of metabolism in animal models and humans. J Intern Med 277:513–527. https://doi.org/10.1111/joim.12347
Education Resources. https://ccb.ucsd.edu/the-bioclock-studio/education-resources/index.html. Accessed 12 Jul 2021
Matsuo T (2003) Control mechanism of the circadian clock for timing of cell division in vivo. Sci 302:255–259. https://doi.org/10.1126/science.1086271
Fu L, Pelicano H, Liu J et al (2002) The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111:41–50
Zhang EE, Liu Y, Dentin R et al (2010) Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med 16:1152–1156. https://doi.org/10.1038/nm.2214
Ozturk N, Lee JH, Gaddameedhi S, Sancar A (2009) Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc Natl Acad Sci 106:2841–2846. https://doi.org/10.1073/pnas.0813028106
Gerhart-Hines Z, Feng D, Emmett MJ et al (2013) The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature 503:410–413. https://doi.org/10.1038/nature12642
Duez H, van der Veen JN, Duhem C et al (2008) Regulation of bile acid synthesis by the nuclear receptor Rev-erbα. Gastroenterology 135:689–698.e5. https://doi.org/10.1053/j.gastro.2008.05.035
Acknowledgements
This work has been supported by Agencia Nacional de Promoción Científica y Técnica (FONCyT, PICT 2016-0187, PICT 2017-631, PICT 2017-0224), Consejo Nacional de Investigaciones Científicas y Tecnológicas de la República Argentina (CONICET) (PIP 2014, PIP 2017), Secretaría de Ciencia y Tecnología de la Universidad Nacional de Córdoba (SeCyT-UNC, Consolidar 2018-2022), and Secretaría de Ciencia y Tecnología de la Universidad Nacional del Sur (PGI-UNS 24B292).
Author information
Authors and Affiliations
Contributions
All authors (MEG, NMM, PMW, and GAS) contributed to the first draft of the manuscript, performed the literature search and data analysis, and critically revised the work. The illustrations were made by PMW and NMM and final edition by NMM. The Table 1 was made by PMW. The last version was revised by MEG, and all authors commented on previous versions of the manuscript and read and approved the final version.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Natalia M. Monjes and Paula M. Wagner contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guido, M.E., Monjes, N.M., Wagner, P.M. et al. Circadian Regulation and Clock-Controlled Mechanisms of Glycerophospholipid Metabolism from Neuronal Cells and Tissues to Fibroblasts. Mol Neurobiol 59, 326–353 (2022). https://doi.org/10.1007/s12035-021-02595-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02595-4