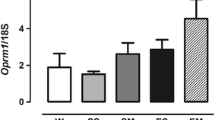
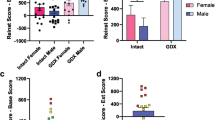
Abstract
Sex differences in opioid analgesia occur in rodents and humans, and could be due to differences in drug and metabolite levels. Thus, we investigated the sex and cycle differences in analgesia (nociception) from oxycodone in rats and related these to sex and cycle differences in brain and plasma oxycodone and metabolite levels. Since numerous opioids are CYP2D enzyme substrates and variation in CYP2D alters opioid drug levels and response, we also initiated studies to see if the sex and cycle differences observed might be due to differences in brain CYP2D activity. Across oxycodone doses, females in diestrus had higher analgesia (using tail flick latency) compared to males and females in estrus; we also demonstrated a direct effect of estrous cycle on analgesia within females. Consistent with the analgesia, females in diestrus had highest brain oxycodone levels (assessed using microdialysis) compared to males and females in estrus. Analgesia correlated with brain oxycodone, but not brain oxymorphone or noroxycodone levels, or plasma drug or metabolite levels. Propranolol (a CYP2D mechanism–based inhibitor), versus vehicle pre-treatments, increased brain oxycodone, and decreased brain oxymorphone/oxycodone drug level ratios (an in vivo CYP2D activity phenotype in the brain) in males and females in estrus, but not in females in diestrus. Brain oxymorphone/oxycodone inversely correlated with analgesia. Together, both sex and estrous cycle impact oxycodone analgesia and brain oxycodone levels, likely through regulation of brain CYP2D oxycodone metabolism. As CYP2D6 is expressed in human brain, perhaps similar sex and cycle influences also occur in humans.





Similar content being viewed by others
Data Availability
Supporting data and material can be found in the additional files and can be requested from the corresponding author.
Code Availability
Not applicable.
References
Degenhardt L, Grebely J, Stone J, Hickman M, Vickerman P, Marshall BDL, Bruneau J, Altice FL et al (2019) Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet 394(10208):1560–1579. https://doi.org/10.1016/S0140-6736(19)32229-9
Wilson N, Kariisa M, Seth P, Smith H, Davis NL (2020) Drug and opioid-involved overdose deaths - United States, 2017–2018. MMWR Morb Mortal Wkly Rep 69(11):290–297. https://doi.org/10.15585/mmwr.mm6911a4
Canada PHAo (2021) Opioids and stimulant-related harms in Canada. Ottawa
Averitt DL, Eidson LN, Doyle HH, Murphy AZ (2019) Neuronal and glial factors contributing to sex differences in opioid modulation of pain. Neuropsychopharmacology 44(1):155–165. https://doi.org/10.1038/s41386-018-0127-4
Bodnar RJ, Kest B (2010) Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Horm Behav 58(1):72–81. https://doi.org/10.1016/j.yhbeh.2009.09.012
Craft RM (2003) Sex differences in opioid analgesia: “from mouse to man.” Clin J Pain 19(3):175–186
Chan S, Edwards SR, Wyse BD, Smith MT (2008) Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat. Clin Exp Pharmacol Physiol 35(3):295–302
Lee CW, Ho IK (2013) Sex differences in opioid analgesia and addiction: interactions among opioid receptors and estrogen receptors. Mol Pain 9:45. https://doi.org/10.1186/1744-8069-9-45
Li J, Xie M, Wang X, Ouyang X, Wan Y, Dong G, Yang Z, Yang J, Yue J (2015) Sex hormones regulate cerebral drug metabolism via brain miRNAs: down-regulation of brain CYP2D by androgens reduces the analgesic effects of tramadol. Br J Pharmacol 172(19):4639–4654. https://doi.org/10.1111/bph.13206
Cepeda MS, Carr DB (2003) Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth Analg 97(5):1464–1468. https://doi.org/10.1213/01.ane.0000080153.36643.83
Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Staud R (2005) Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain 6(2):116–124. https://doi.org/10.1016/j.jpain.2004.11.005
Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Teppema L et al (2000) Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology 93(5):1245–1254. https://doi.org/10.1097/00000542-200011000-00018 (discussion 1246A)
Cicero TJ, Nock B, Meyer ER (1996) Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther 279(2):767–773
Kanarek RB, Homoleski B (2000) Modulation of morphine-induced antinociception by palatable solutions in male and female rats. Pharmacol Biochem Behav 66(3):653–659. https://doi.org/10.1016/s0091-3057(00)00251-3
Holtman JR, Wala EP (2006) Characterization of the antinociceptive effect of oxycodone in male and female rats. Pharmacol Biochem Behav 83(1):100–108. https://doi.org/10.1016/j.pbb.2005.12.013
Peckham EM, Traynor JR (2006) Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. J Pharmacol Exp Ther 316(3):1195–1201. https://doi.org/10.1124/jpet.105.094276
Collins D, Reed B, Zhang Y, Kreek MJ (2016) Sex differences in responsiveness to the prescription opioid oxycodone in mice. Pharmacol Biochem Behav 148:99–105. https://doi.org/10.1016/j.pbb.2016.06.006
Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD (2006) Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther 79(5):461–479. https://doi.org/10.1016/j.clpt.2006.01.009
Salmin SF, Giroux MC, Vachon P, Beaudry F (2017) In vitro metabolism of specific CYP2D and CYP3A opioid substrates using rat liver S9 fractions and mass spectrometry reveal a severe metabolic impairment with increasing age. Biomed Chromatogr 31 (2). doi:https://doi.org/10.1002/bmc.3786
McMillan DM, Miksys S, Tyndale RF (2019) Rat brain CYP2D activity alters in vivo central oxycodone metabolism, levels and resulting analgesia. Addict Biol 24(2):228–238. https://doi.org/10.1111/adb.12590
Bostrom E, Simonsson US, Hammarlund-Udenaes M (2006) In vivo blood-brain barrier transport of oxycodone in the rat: indications for active influx and implications for pharmacokinetics/pharmacodynamics. Drug Metab Dispos 34(9):1624–1631. https://doi.org/10.1124/dmd.106.009746
Cora MC, Kooistra L, Travlos G (2015) Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol 43(6):776–793. https://doi.org/10.1177/0192623315570339
Le Bars D, Gozariu M, Cadden SW (2001) Animal models of nociception. Pharmacol Rev 53(4):597–652
McMillan DM, Tyndale RF (2015) Nicotine increases codeine analgesia through the induction of brain CYP2D and central activation of codeine to morphine. Neuropsychopharmacology 40(7):1804–1812. https://doi.org/10.1038/npp.2015.32
Rowland K, Yeo WW, Ellis SW, Chadwick IG, Haq I, Lennard MS, Jackson PR, Ramsay LE et al (1994) Inhibition of CYP2D6 activity by treatment with propranolol and the role of 4-hydroxy propranolol. Br J Clin Pharmacol 38(1):9–14. https://doi.org/10.1111/j.1365-2125.1994.tb04315.x
Masubuchi Y, Narimatsu S, Hosokawa S, Suzuki T (1994) Role of the CYP2D subfamily in metabolism-dependent covalent binding of propranolol to liver microsomal protein in rats. Biochem Pharmacol 48(10):1891–1898. https://doi.org/10.1016/0006-2952(94)90587-8
Miksys S, Wadji FB, Tolledo EC, Remington G, Nobrega JN, Tyndale RF (2017) Rat brain CYP2D enzymatic metabolism alters acute and chronic haloperidol side-effects by different mechanisms. Prog Neuropsychopharmacol Biol Psychiatry 78:140–148. https://doi.org/10.1016/j.pnpbp.2017.04.030
Li J, Wan Y, Na S, Liu X, Dong G, Yang Z, Yang J, Yue J (2015) Sex-dependent regulation of hepatic CYP3A by growth hormone: roles of HNF6, C/EBPα, and RXRα. Biochem Pharmacol 93(1):92–103. https://doi.org/10.1016/j.bcp.2014.10.010
Waxman DJ, Ram PA, Pampori NA, Shapiro BH (1995) Growth hormone regulation of male-specific rat liver P450s 2A2 and 3A2: induction by intermittent growth hormone pulses in male but not female rats rendered growth hormone deficient by neonatal monosodium glutamate. Mol Pharmacol 48(5):790–797
De Gregori S, De Gregori M, Ranzani GN, Allegri M, Minella C, Regazzi M (2012) Morphine metabolism, transport and brain disposition. Metab Brain Dis 27(1):1–5. https://doi.org/10.1007/s11011-011-9274-6
Kaiko RF, Benziger DP, Fitzmartin RD, Burke BE, Reder RF, Goldenheim PD (1996) Pharmacokinetic-pharmacodynamic relationships of controlled-release oxycodone. Clin Pharmacol Ther 59(1):52–61. https://doi.org/10.1016/S0009-9236(96)90024-7
Cleary J, Mikus G, Somogyi A, Bochner F (1994) The influence of pharmacogenetics on opioid analgesia: studies with codeine and oxycodone in the Sprague-Dawley/Dark Agouti rat model. J Pharmacol Exp Ther 271(3):1528–1534
Gronlund J, Saari TI, Hagelberg NM, Neuvonen PJ, Olkkola KT, Laine K (2010) Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br J Clin Pharmacol 70(1):78–87. https://doi.org/10.1111/j.1365-2125.2010.03653.x
Klimas R, Witticke D, El Fallah S, Mikus G (2013) Contribution of oxycodone and its metabolites to the overall analgesic effect after oxycodone administration. Expert Opin Drug Metab Toxicol 9(5):517–528. https://doi.org/10.1517/17425255.2013.779669
Lalovic B, Phillips B, Risler LL, Howald W, Shen DD (2004) Quantitative contribution of CYP2D6 and CYP3A to oxycodone metabolism in human liver and intestinal microsomes. Drug Metab Dispos 32(4):447–454. https://doi.org/10.1124/dmd.32.4.447
Sadiq MW, Boström E, Keizer R, Björkman S, Hammarlund-Udenaes M (2013) Oxymorphone active uptake at the blood-brain barrier and population modeling of its pharmacokinetic-pharmacodynamic relationship. J Pharm Sci 102(9):3320–3331. https://doi.org/10.1002/jps.23492
Grossman ML, Basbaum AI, Fields HL (1982) Afferent and efferent connections of the rat tail flick reflex (a model used to analyze pain control mechanisms). J Comp Neurol 206(1):9–16. https://doi.org/10.1002/cne.902060103
Acknowledgements
We would like to thank Dr. Bin Zhao for his invaluable technical assistance with the LC-MS analyses, Dr. Ahmed El-Sherbeni for assistance with MD calculations, and Fariba Baghai Wadji for her expert support with stereotaxic surgeries and microdialysis animal procedures.
Funding
This work was supported by the R01 grant from the National Institutes of Health (DA043526); Canada Research Chairs program (Dr. Tyndale, the Canada Research Chair in Pharmacogenomics), a Canadian Institutes of Health Research (Foundation Grant FDN-154294); the Centre for Addiction and Mental Health; and the CAMH Foundation.
Author information
Authors and Affiliations
Contributions
N.A., S.M, and R.F.T designed all the research experiments. N.A. did the stereotaxic surgeries and ran all the experiments. N.A. analyzed the data. N.A and R.F.T wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Experiments were performed in accordance with the NIH guidelines for the care and use of laboratory animals, and with approval of the University of Toronto Animal Care Committee.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
Dr. Rachel F Tyndale has consulted for Quinn Emanuel and Ethismos on topics unrelated to this current work. Nicole Arguelles and Dr. Sharon Miksys have no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arguelles, N., Miksys, S. & Tyndale, R.F. Sex and Estrous Cycle Differences in Analgesia and Brain Oxycodone Levels. Mol Neurobiol 58, 6540–6551 (2021). https://doi.org/10.1007/s12035-021-02560-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02560-1